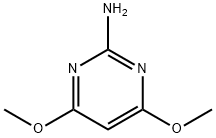
2-Amino-4,6-dimethoxypyrimidine: Eco-Friendly Synthesis and Enhancing Role in Electrolytes
2-amino-4,6-dimethoxypyrimidine (ADMP) is an important intermediate for the synthesis of sulfonylurea herbicides. As the most critical step in the ADMP synthesis, ADMP prepared by cyclization of 3-amino-3-methoxy-N-cyano-2-propaneamidine (AMCP) are rarely reported, especially its reaction mechanism.

Synthesis of 2-amino-4,6-dimethoxypyrimidine with dimethyl
2-amino-4,6-dimethoxypyrimidine(ADM) is one of the most important pesticide intermediates, which has been widely used for the synthesis of some effective and environmentally friendly pesticides, such as sulfonylurea herbicides. Traditionally, ADM has been obtained through three steps, first using guanidine nitrate, diethyl malonate to get 2-amino-4,6-dihydroxypyrimidine(ADH), then reacting with phosphorus oxychloride to get 2-amino-4,6-dichloropyrimidine(ADC), finally getting 2-Amino-4,6-dimethoxypyrimidine by methylation. the reaction processes are fairly complicated, and the final products are difficult to deal with because of the presence of wastes. So, it is necessary to search out a less-polluting and more environmentally friendly synthetic route for ADM. It has also been reported that veratrole was obtained in a high yield by the vapor phase methylation of catechol with DMC when alumina was loaded with various alkali metal compounds. In this work, 2-Amino-4,6-dimethoxypyrimidine was prepared from ADH in the presence of K2CO3 and PTC, using DMC as methylating agent instead of conventional toxic reagents (such as haloalkane and dimethyl sulfate, etc.). The optimum reaction conditions were obtained considering these influencing factors such as kinds of PTC, reaction time, reaction temperature, reactant ratio, and so on. The results show that the synthesis conditions are mild and easy to control.[1]
The effect of reaction temperature on the conversion of ADH and selectivity of 2-Amino-4,6-dimethoxypyrimidine was examined at a DMC/ADH ratio of 5; the results are shown. In the temperature range studied, the best conversion was 87.5 %. The selectivity of ADM increased with the enhancement of reaction temperature from 130 to 150 °C. However, due to some side reactions, the selectivity of 2-Amino-4,6-dimethoxypyrimidine did not show a continuous increase when the reaction temperature was enhanced. For, on the one hand, DMC broke down easily under alkaline conditions at higher temperature, while on the other hand, quaternary ammonium PTC have a poor thermal stability. K2CO3/TBAB showed high catalytic activity and selectivity for the O-methylation of ADH with DMC to yield ADM. Thus, the highest conversion (87.7 %) of ADH and selectivity (40.5 %) of 2-Amino-4,6-dimethoxypyrimidine were achieved at 150 °C for 10 h with a ADH/DMC/TBAB/K2CO3 ratio of 1/5/0.1/3. Compared with the traditional synthesis methods which are bad for the environment, the synthetic route reported (which has not previously been reported) was more ecofriendly, in accordance with the development direction of green chemistry.
Effects of 2-Amino-4,6-Dimethoxypyrimidine on PVDF/KI/I2-Based Solid Polymer Electrolytes
Different weight ratios of 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based solid polymer electrolytes (SPEs) were prepared by solution-casting method. The prepared these doped PVDF/KI/I2-based SPEs were characterized by powder x-ray diffraction (PXRD) analysis, AC impedance analysis and scanning electron microscopy (SEM) analysis. The crystallinity of 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based SPEs was confirmed by PXRD measurement. The ionic conductivity of 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based SPEs was calculated using AC impedance analysis. The ionic conductivity values of different weight ratios of these PVDF/KI/I2-based SPEs are 5.50 × 10−6 S cm−1, 1.74 × 10−5 S cm−1, 4.91 × 10−5 S cm−1, 2.04 × 10−5 S cm−1, 1.58 × 10−5 S cm−1 and 1.04 × 10−5 S cm−1, respectively.[2]
Ionic conductivity studies revealed that the 20% these doped PVDF/KI/I2-based SPE showed the highest ionic conductivity value. The SEM images show the surface morphology of 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based SPEs. The power conversion efficiency (PCE) of DSSCs utilizing different weight ratios (0%, 10%, 20%, 30%, 40% and 50%) of these doped PVDF/KI/I2-based SPEs are 1.4%, 2.0%, 2.5%, 2.3%, 1.9% and 1.6%, respectively. These results revealed that the DSSC using 20% 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based SPE exhibited the highest PCE. The lowest crystallinity is shown in the 20% doped PVDF/KI/I2-based SPE. The surface morphology of 20% doped PVDF/KI/I2-based SPE has the smallest spherical particles with voids. The 20% 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based SPE has the highest ionic con ductivity value of 4.91 9 10 5 Scm1. The DSSC with 20% 2-amino-4,6-dimethoxypyrimidine-doped PVDF/KI/I2-based SPE achieved the highest PCE of 2.5%
References
[1]Xiong, Z., Zhou, M., & Xiao, G. (2013). Synthesis of 2-amino-4,6-dimethoxypyrimidine with dimethyl carbonate as methylating agent. Research on Chemical Intermediates, 40, 1789-1797.
[2]Sundaramoorthy, K., Muthu, S. P., & Perumalsamy, R. (2020). Effects of 2-Amino-4,6-Dimethoxypyrimidine on PVDF/KI/I₂-Based Solid Polymer Electrolytes for Dye-Sensitized Solar Cell Application. Journal of Electronic Materials, 49, 3728-3734.
You may like
See also
Lastest Price from 2-Amino-4,6-dimethoxypyrimidine manufacturers

US $10.00/KG2025-04-21
- CAS:
- 36315-01-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt
US $1.10/g2025-04-17
- CAS:
- 36315-01-2
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min