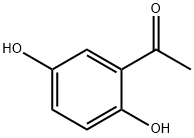
2',5'-Dihydroxyacetophenone: Hypouricemic, Anti-Melanogenic, and Anti-Multiple Myeloma Properties
2',5'-Dihydroxyacetophenone, isolated from Rehmannia glutinosa, inhibits the production of inflammatory mediators in activated macrophages by blocking the ERK1/2 and NF-κB signaling pathways. 2',5'-Dihydroxyacetophenone (Quinacetophenone) is an uncompetitive inhibitor of murine tyrosinase (K (I) 0.28mm), it strongly inhibits both melanogenesis and cellular tyrosinase activity in vitro in 3-isobutyl-1-methylxanthin-stimulated B16 mouse melanoma cells or in vivo in zebrafish and mouse models.

Hypouricemic Effect of 2',5'-Dihydroxyacetophenone
Uric acid, as a sparingly soluble compound, is the end product of purine catabolism in humans, since the silent mutation of uricase in human evolution. It is accumulated in tissues and extracellular fluid in the appearance of monosodium urate (MSU). Previously, we reported that G. applanatum resisted HUA through enhancing uric acid excretion by downregulating GLUT9 (glucose transporter 9) and upregulating OAT1 (organic anion transporter 1) and exhibited some nephron- and liver-protective effects. Nonetheless, the bioactive mechanism against HUA is not yet clear. Given that, computational virtual screening was performed using the XOD structure, and 2',5'-Dihydroxyacetophenone (DHAP) ranked high. Firstly, XOD inhibitory effect of DHAP was assayed, in vitro, to verify the prediction. Then, its hypouricemic effects were examined on hyperuricemic mice, wherein, SUA, BUN (blood urea nitrogen) and creatinine were recorded. RNA and protein expressions of OAT1, GLUT9, URAT1 (uric acid transporter 1), and CNT2 (gastrointestinal concentrative nucleoside transporter 2) were examined by RT-PCR and Western blot. Also, XOD activities, in vitro, in serum, were tested by enzyme-linked immunosorbent assay (ELISA) method. Due to its inhibition against XOD, molecular docking was conducted to explore the details of the binding of 2',5'-Dihydroxyacetophenone to XOD.[1]
DHAP is known to induce cell death through inducing apoptosis via regulating the Ras–mitogen-activated protein kinase (MAPK) activation pathway. Moreover, an anti-inflammatory effect was also observed for 2',5'-Dihydroxyacetophenone. DHAP did show an excellent hypouricemic effect through inhibiting XOD, suppressing URAT1, GLUT9, and CNT2, and elevating OAT1, which was comparable to allopurinol and benzbromarone. Renal injuries of hyperuricemic mice were alleviated by 2',5'-Dihydroxyacetophenone. Also, anti-inflammatory effects and little toxicity were observed. Due to its excellent hypouricemic effect in vivo and vitro, use of DHAP as a template to design and synthesize analogs with improved pharmacological properties against hyperuricemia or even metabolic syndrome should be underway. This research provided evidence that DHAP was a promising hypouricemic agent, which may associate with several pathways. Further research should be focused on its structure modification and optimization. In summary, we report the hypouricemic effect of 2',5'-Dihydroxyacetophenone, a constituent screened from G. applanatum in silico. Its hypouricemic effect was mediated by decreasing XOD activities, along with its upregulation of OAT1 and downregulation of GLUT9, URAT1, and CNT2.
2',5'-Dihydroxyacetophenone isolated from Ganoderma cochlear
Melanin is a pigment that exists on the surface of the skin and affects the colour of the skin in organisms. It can protect the skin from various injuries under ultraviolet light. In the previous study, a natural source of 2',5'-Dihydroxyacetophenone was found from the wild Ganoderma cochlear. Considering the compound is structurally similar to hydroquinone and is likely to have a whitening and freckle-removing effect, it was investigated in terms of inhibiting melanogenesis. Furthermore, structural analogs of 2',5'-Dihydroxyacetophenone were systematically investigated for their melanogenesis-inhibitory properties. Through comparative analysis, compounds exhibiting significant inhibitory effects were identified. This study was designed to provide validated research foundations for the development of effective melanin synthesis inhibitors based on the 2',5'-Dihydroxyacetophenone pharmacophore.[2]
2,5-Dihydroxyacetophenone was demonstrated to effectively inhibit tyrosinase activity and reduce melanin production in both murine and zebrafish models, establishing it as a potent yet low-toxicity natural anti-melanogenic agent. In this study, a diverse series of structurally modified analogs, based on the 2',5'-Dihydroxyacetophenone scaffold, were synthesised or commercially procured for comprehensive evaluation. Through systematic enzyme assays and zebrafish testing, fourteen compounds were identified as effective melanogenesis inhibitors. Among these, some exhibited the most pronounced inhibitory effects, providing a robust theoretical foundation for future development of depigmenting agents.
2',5'-Dihydroxyacetophenone Induces Apoptosis of Multiple Myeloma Cells
Radix rehmanniae is obtained from the root of the herbaceous plant Rehmannia glutinosa Libosch. It is a traditional Chinese medicinal herb and has been found to have biological properties like anti-inflammatory and wound-healing effects and the ability to attenuate diabetic nephropathy. 2',5'-Dihydroxyacetophenone (DHAP) is a one of the bioactive compounds isolated from Radix rehmanniae preparata, the steamed root of Radix rehmanniae, and has also been reported to have anti-inflammatory properties through the modulation of nuclear factor kappa B (NF-κB) pathway-mediated inflammatory responses in the activated macrophages. For this study, we attempted to determine if DHAP’s anti-cancer properties stem from the triggering of apoptosis in the multiple myeloma cell lines. We discovered that DHAP effectively inhibited multiple myeloma cell growth, induced apoptosis, and upregulated MAPK signaling pathways in vitro. In addition, 2',5'-Dihydroxyacetophenone exhibited synergistic effects when combined with the proteasome inhibitor, bortezomib. Our results therefore demonstrate the potential effectiveness of DHAP for the treatment of multiple myeloma.[3]
The objective of this research was to determine the anti-cancer properties of DHAP in multiple myeloma cells. It was observed that 2',5'-Dihydroxyacetophenone downregulated the expression of certain gene products, including Bcl-xl, Bcl-2, Mcl-1, Survivin, IAP1, Cyclin D1, Cyclin E, MMP-9, and COX-2, with concomitant up-regulation of Bax and p21 expression. DHAP caused G2/M phase arrest, inhibition of proliferation, induction of apoptosis, and led to activation of caspase-3, caspase-8, and caspase-9. We also found that 2',5'-Dihydroxyacetophenone induced the activation of JNK, p38, and ERK, and caused substantial potentiation of bortezomib’s apoptotic effects in U266 cells. Furthermore, combination treatment resulted in greater phosphorylation of JNK, compared with 2',5'-Dihydroxyacetophenone or bortezomib alone. Overall, our results demonstrate that DHAP inhibited the proliferation of tumor cells by causing apoptosis and G2/M phase arrest. DHAP-induced cell cycle arrest and apoptosis were linked to the activation of the MAPK pathway.
References
[1]Liang D, Yong T, Chen S, Xie Y, Chen D, Zhou X, Li D, Li M, Su L, Zuo D. Hypouricemic Effect of 2,5-Dihydroxyacetophenone, a Computational Screened Bioactive Compound from Ganoderma applanatum, on Hyperuricemic Mice. Int J Mol Sci. 2018 May 7;19(5):1394. doi: 10.3390/ijms19051394. PMID: 29735945; PMCID: PMC5983617.
[2]Ning M, Liu XC, He M, Peng XR, Qiu MH. 2,5-Dihydroxyphenylethanone: an anti-melanogenic bioactive compound isolated from Ganoderma cochlear. J Enzyme Inhib Med Chem. 2025 Dec;40(1):2495364. doi: 10.1080/14756366.2025.2495364. Epub 2025 Apr 29. PMID: 40302176; PMCID: PMC12044912.
[3]Ko JH, Lee JH, Jung SH, Lee SG, Chinnathambi A, Alharbi SA, Yang WM, Um JY, Sethi G, Ahn KS. 2,5-Dihydroxyacetophenone Induces Apoptosis of Multiple Myeloma Cells by Regulating the MAPK Activation Pathway. Molecules. 2017 Jul 11;22(7):1157. doi: 10.3390/molecules22071157. PMID: 28696369; PMCID: PMC6152349.
You may like
See also
Lastest Price from 2',5'-Dihydroxyacetophenone manufacturers

US $10.00/kg2025-04-21
- CAS:
- 490-78-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 mt

US $45.00/kg2025-04-21
- CAS:
- 490-78-8
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons