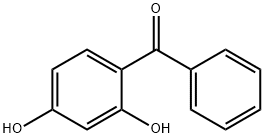
2,4-Dihydroxybenzophenone: an efficient UV resistance
2,4-Dihydroxybenzophenone (benzophenone-1; BP-1) is an UV stabilizer primarily used to prevent polymer degradation and deterioration in quality due to UV irradiation. Recently, BP-1 has been reported to bioaccumulate in human bodies by absorption through the skin and has the potential to induce health problems including endocrine disruption.

Synthesis of 2,4-Dihydroxybenzophenone
Add 110kg of resorcinol, 150kg of water, and 30kg of ethanol to a reactor equipped with a gas absorption device, turn on the stirring to make the raw materials evenly mixed, heat up to 40°C, start dripping 205kg of trichlorotoluene, the dripping time is 3h After the dripping is completed, the temperature is raised to 50°C, and the reaction is kept for 1 hour. After the reaction is completed, it is cooled to room temperature, water is added to the reaction solution, and centrifuged.Obtain 2,4-dihydroxybenzophenone solid and mother liquor containing 2,4-dihydroxybenzophenone and by-products benzoic acid and hydrogen chloride;(2) Concentrate the mother liquor and recover ethanol to be used in the acylation reaction in step (1). After the mother liquor is cooled to room temperature, it is filtered. The filter cake is 2,4-dihydroxybenzophenone, and the filtrate contains benzene. Aqueous solutions of formic acid and hydrogen chloride;(3) Add 10% mass fraction of sodium hydroxide solution to the filtrate, adjust the pH of the solution to 8, add 2kg of activated carbon, and stir for 30-60 minutes, then separate the solution from the activated carbon to obtain an aqueous solution of sodium benzoate;(4) Add a 10% hydrochloric acid solution to the sodium benzoate aqueous solution, adjust the pH of the solution to 1, stir, filter, and dry to obtain benzoic acid.The 2,4-dihydroxybenzophenone in step (1) and step (2) are combined and dried to obtain 209.1 kg of 2,4-dihydroxybenzophenone with a yield of 97.6% and a GC content of 99.3%. HPLC determined that the ethanol content in the final mother liquor was less than 1%, the 2,4-dihydroxybenzophenone content was less than 0.4%, and the benzoic acid content was less than 0.5%.[1]
Protective effects of 2,4-dihydroxybenzophenone
2,4-dihydroxybenzophenone (BP-1), a benzophenone derivative, is often used as an ultraviolet light absorbent. BP-1 has also been used in some products such as nail polish, polish remover, shaving cream, body cleanser and so on. Previous researches about BP-1 mainly focused on its estrogenic activities. Matsumoto et al demonstrated that BP-1 exhibited estrogenic activities by estrogen receptor using MCF-7 (a human breast cancer cell line) cell proliferation assay. In this study, we examined the effects of BP-1 in APAP-induced hepatotoxicity in mice.[2]
Twenty-four hours after a single dose of APAP (350 mg/kg body weight), serum levels of ALT, AST and LDH increased significantly (P < 0.05), showing characteristic acute liver injury induced by APAP. Compared with APAP group, 2,4-Dihydroxybenzophenone groups lowered serum levels of ALT, AST and LDH dramatically. At a dose of 200 mg/kg body weight, the difference was not significant; at doses of 400 and 800 mg/kg body weight, there were significant differences between 2,4-Dihydroxybenzophenone groups and APAP group (P < 0.05). The effect of 2,4-Dihydroxybenzophenone was dose-dependent and serum levels of ALT, AST and LDH in the highest dose groups were comparable to that of vehicle control group (P > 0.05). Compared with vehicle control group, MDA content in liver tissue of APAP alone group increased while the ratio of GSH/GSSG decreased dramatically (P < 0.05), indicating an oxidation stress and depletion of GSH. With increasing dose of 2,4-Dihydroxybenzophenone, MDA level decreased and GSH/GSSG ratio increased. In the highest dose group (800 mg/kg body weight), the MDA and ratio of GSH/GSSG were close to the normal level as shown in vehicle control group. Observed by naked eyes, the livers of vehicle control group were deep red, moist, glossy and resilient. In APAP group, the livers lost luster and yellow necrosis foci were often found on the surface. Liver injury of 2,4-Dihydroxybenzophenone pretreated mice was attenuated dramatically in a dose-dependent manner.
Under light microscope, liver lobular structures in vehicle control group were clear and regular, and single layer of hepatocytes arranged around the central vein in a radical pattern. There were abundant basophilic granular cytoplasms in the hepatocytes. In APAP-intoxicated mice, normal liver lobular structures were damaged and collapsed. The hepatocytes showed vacuolization, sinusoidal dilation and congestion. Infiltration of inflammatory cells and loss of cell boundaries were also observed. Pre-administration of 2,4-Dihydroxybenzophenone showed mild injuries in a dose-related manner. BP-1 at 200 mg/kg could not effectively prevent the damage. 2,4-Dihydroxybenzophenone showed a broad protective effect in hepatotoxic chemicals-induced acute liver injury, implying that it may target on the oxidative stress, which was the key event in liver injuries caused by these hepatotoxic chemicals. The results demonstrated that, MDA levels in APAP-intoxicated mice increased obviously and GSH/GSSG ratio decreased, implying the existence of oxidative damage and depletion of GSH. However, pre-administration of 2,4-Dihydroxybenzophenone decreased the MDA level and increased GSH/GSSG ratio in a dose-dependent manner. The results indicated that the hepatoprotective effect of 2,4-Dihydroxybenzophenone was associated with its antioxidant activity.
Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone
To acquire solid evidence of substituent effects on benzophenone, Scientists chose 2,4-dihydroxybenzophenone for such a goal. In this study, nine compounds of 2,4-dihydroxy dibenzophenone with different substituents were synthesized and their ultraviolet absorption performance and UV resistance of cotton fabric and polyester analyzed. Moreover, the dyeing rate of each compound on cotton fabric and polyester were tested, respectively. The outcome of the present study is anticipated that a research basis for the development and design of benzophenone UV absorbents.[3]
The UV absorption spectra of the nine compounds were collected at room temperature on a UV-1800 UV-vis spectrometer having 2,4-dihydroxy dibenzophenone in a concentration range of 10−3 to 10−5 mol/L. The ethanol used in the experiments was of spectroscopic grade purchased from Aladdin Industrial Corporation (Shanghai, China) and was used as purchased. For comparison purposes the maximum absorbance is normalized for all measurements. The UPF values of cotton fabric and polyester increase with the increase in compound concentration. For cotton fabric, when the concentration of UV absorbent is 2 × 10−2 mol·L−1, 2,4-dihydroxybenzophenone has the best UV resistance of 143.2, but its UV resistance for polyester is slightly lower than that endowed with the substituted groups. The presence of the methoxyl group does not significantly improve the UV resistance of polyester. It is envisaged that through the selection of substituent, the UV resistance properties of 2,4-dihydroxybenzophenone can be tuned for specific applications.
Substituent effects on the ultraviolet absorption properties of 2,4-dihydroxy dibenzophenone were investigated experimentally. Nine compounds of 2,4-dihydroxy dibenzophenone with different substituents were prepared by a solvent-free reaction of benzoyl chloride. The maximum absorption wavelength (λmax) of these samples was measured, and their UV resistance properties in cotton fabric as well as in polyester were determined. The results show that the λmax is dependent on the substituents at the benzylidene ring, and both electron donating substituents and electron withdrawing substituents cause a bathochromic shift. The UV resistance of fabric increases with the increase in compound concentration. The dyeing rate of each compound on polyester was higher than that of cotton. On cotton fabric, the dyeing rate of 2,4-dihydroxybenzophenone was the highest, 77.8%. On polyester, that of 2,4-dihydroxy-4′-ethyl dibenzophenone was the highest, 84.1%. The study provides new insights into the effect of substituents on the properties of 2,4-dihydroxy dibenzophenone that are related to the whitening of cotton and polyester materials.
References
[1] JIANGSU ZIQI CHEMICAL TECH - CN112142576, 2020, A
[2] He YY, Zhang BX, Jia FL. Protective effects of 2,4-dihydroxybenzophenone against acetaminophen-induced hepatotoxicity in mice. World J Gastroenterol. 2011 Jun 7;17(21):2663-6.
[3] Wu F, Tan S, Fang Z, Deng J, He Z, Huang C, Au C, Yi B. Substituent Effects on the Ultraviolet Absorption Properties of 2,4-Dihydroxy Dibenzophenone. Molecules. 2022 Nov 24;27(23):8169.
See also
Lastest Price from 2,4-Dihydroxybenzophenone manufacturers

US $20.00/box2025-07-23
- CAS:
- 131-56-6
- Min. Order:
- 1box
- Purity:
- 99
- Supply Ability:
- in stock

US $0.00-0.00/KG2025-07-05
- CAS:
- 131-56-6
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10000KGS