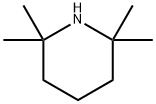
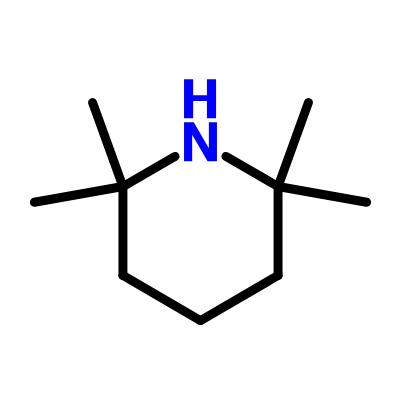
2,2,6,6-Tetramethylpiperidine: applications and safety
General Description
2,2,6,6-Tetramethylpiperidine (TMP) has several applications. It is used as a catalyst in the chlorination of phenols, exhibiting ortho selectivity, meaning that it prefers to substitute at the ortho position of the phenolic ring. It can also act as a metabolic intermediate, undergoing heme-catalyzed metabolism in human liver microsomes. This metabolism results in the formation of 2,2-dimethylpyrrolidine (2,2-DMPy). Additionally, TMP and its derivatives have shown potential as antioxidants, effectively scavenging reactive oxygen species and inhibiting the generation of hydroxyl radicals. However, TMP should be handled with caution as it is flammable, toxic if swallowed, and can cause skin burns and eye damage. Proper safety precautions should be taken when handling this compound.

Figure 1. 2,2,6,6-Tetramethylpiperidine
Applications
Catalysts
2,2,6,6-Tetramethylpiperidine finds application in the catalytic chlorination of phenols using SO2Cl2 in aromatic solvents. Compared to primary and less hindered secondary amine catalysts, TMP-catalyzed chlorinations exhibit greater ortho selectivity. This means that the chlorine atom tends to preferentially substitute at the ortho position of the phenolic ring. Even electron-deficient phenols like 2-hydroxybenzaldehyde and 2'-hydroxyacetophenone can be ortho chlorinated successfully. Interestingly, the ortho selectivity increases with higher reaction temperatures. On the other hand, when tetraalkylammonium chloride is used as the catalyst, the chlorinations are moderately para selective. This means that the chlorine atom substitutes predominantly at the para position of the phenolic ring. The choice between 2,2,6,6-tetramethylpiperidine and tetraalkylammonium chloride as a catalyst depends on the desired regioselectivity (ortho or para) of the chlorination reaction. 1
Metabolic intermediates
2,2,6,6-Tetramethylpiperidine has been found to have applications in the metabolism of certain compounds. In particular, research has shown that 2,2,6,6-TMP can undergo a unique cytochrome P450 (CYP) catalyzed metabolism in human liver microsomes. This metabolism results in the formation of a ring-contracted 2,2-dimethylpyrrolidine (2,2-DMPy). In this process, evidence suggests that 2,2,6,6-Tetramethylpiperidine hydroxylamines and their one-electron oxidation products, known as nitroxide radicals, act as intermediates. These nitroxide radicals can be converted to their corresponding 2,2-DMPy metabolites by "inactivated CYP3A4" as well as other heme proteins and hemin. Therefore, it is believed that this metabolism is heme-catalyzed. During the conversion of nitroxide radicals to 2,2-DMPy products, acetone is generated, indicating that the three-carbon unit from 2,2,6,6-TMP is lost as acetone. Additionally, another pathway was observed, where an intermediate incorporating two oxygen atoms from water before collapsing to the 2,2-DMPy product was formed. Based on these observations, a proposed mechanism suggests that the interaction of nitroxide radicals with heme iron leads to the formation of a perferryl-oxygen complex. This complex then reacts with the 2,2,6,6-Tetramethylpiperidine radical, causing the contraction of the piperidine ring and subsequent oxidation to an exocyclic iminium ion. Water capture and N-dealkylation then eliminate the three-carbon unit. 2
Antioxidants
2,2,6,6-Tetramethylpiperidine has shown potential applications as antioxidants. Two new derivatives of 2,2,6,6-TMP, namely Tempace and Troxyl, were synthesized and evaluated for their abilities to scavenge superoxide, inhibit iron and ascorbate-driven Fenton reactions, and act as radioprotectors. The results of the study indicate that Tempace and Troxyl exhibit promising antioxidant properties. They were found to effectively scavenge superoxide, which is a reactive oxygen species involved in oxidative stress. Additionally, these compounds demonstrated the ability to inhibit the iron and ascorbate-driven Fenton reaction, which is responsible for the generation of highly reactive hydroxyl radicals. Furthermore, the potential for one-electron oxidation of Tempace and Troxyl by heme-ferryl species was investigated. This highlights the interaction of these compounds with oxidized heme molecules, suggesting their involvement in redox processes. Given their antioxidant and radioprotective properties, Tempace and Troxyl present a foundation for further research and exploration in various pharmacological applications. 3
Safety
2,2,6,6-Tetramethylpiperidine is a chemical compound with various safety considerations. It is classified as flammable, toxic if swallowed, and harmful if swallowed. It can cause skin burns, eye damage, and respiratory irritation. To ensure safety, keep the substance away from heat, sparks, and open flames. Smoking should be prohibited. The container should be tightly closed and grounded. Avoid inhaling any associated dust, fumes, or vapors. Thoroughly wash hands and skin after handling and avoid eating, drinking, or smoking. Wear protective gloves, clothing, eye protection, and face protection. In case of emergencies, seek immediate medical assistance if swallowed or exposed to eyes. Rinse eyes with water and remove contact lenses if present. If feeling unwell, seek medical attention. If a fire occurs, use appropriate extinguishing media and wear suitable protective clothing. 4
Reference
1. Saper NI, Snider BB. 2,2,6,6-Tetramethylpiperidine-catalyzed, ortho-selective chlorination of phenols by sulfuryl chloride. J Org Chem, 2014, 79(2):809-813.
2. Yin W, Mitra K, Stearns RA, Baillie TA, Kumar S. Conversion of the 2,2,6,6-tetramethylpiperidine moiety to a 2,2-dimethylpyrrolidine by cytochrome P450: evidence for a mechanism involving nitroxide radicals and heme iron. Biochemistry, 2004, 43(18):5455-5466.
3. Metodiewa D, Skolimowski J, Karolczak S. Tempace and troxyl-novel synthesized 2,2,6,6-tetramethylpiperidine derivatives as antioxidants and radioprotectors. Biochem Mol Biol Int, 1996, 40(6):1211-1219.
4. Chemical Safety Data Sheet MSDS/SDS: 2,2,6,6-Tetramethylpiperidine. Chemical Book, 2023, CBnumber: CB3320372.
Related articles And Qustion
See also
Lastest Price from 2,2,6,6-Tetramethylpiperidine manufacturers

US $0.00/KG2025-04-16
- CAS:
- 768-66-1
- Min. Order:
- 25KG
- Purity:
- ≥99.0%
- Supply Ability:
- 5tons
US $0.00-0.00/KG2025-04-04
- CAS:
- 768-66-1
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton