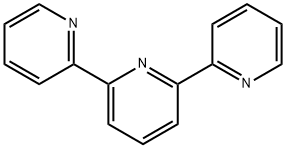
2,2′:6′,2″-terpyridine-Application
Feb 18,2020
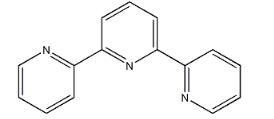
As an NNN-tridentate ligand, the 2,2′:6′,2″-terpyridine plays an important role in coordination chemistry. With three coordination sites and low LUMO, terpyridine and its derivatives are one of the typical Pincer ligand and/or non-innocent ligands in transition metal catalysis. Interesting catalytic reactivities have been obtained with these tpy-metal complexes targeting some challenging transformations, such as C–C bond formation and hydrofunctionalization[1]. The 2,2':6':2''-terpyridine ligand has literally shaped the coordination chemistry of transition metal complexes in a plethora of fields. Expansion of the ligand bite by amine functionalities between the pyridine units in the tridentate N,N'-dimethyl-N,N'-dipyridine-2-ylpyridine- 2,6-diamine ligand (ddpd) modifies the properties of corresponding transition metal complexes, comprising redox chemistry, molecular dynamics, magnetism and luminescence. The origins of these differences between ddpd and tpy complexes will be elucidated and comprehensively summarized with respect to first row transition metal complexes with d2-d10 electron configurations[2]. A series of triimine ligands incorporating 2,2′:6′,2″-terpyridine (terpy) as well as its corresponding Re(I) tricarbonyl complexes were successfully designed and synthesized to investigate the impact of the peripheral rings and phenyl– or naphthyl–based substituents attached to the triimine skeleton on their photophysical and electroluminescent properties.
DSC investigations showed that both the free ligands and Re(I) complexes melted at high temperature (164–309 °C) and some of them are able to form amorphous materials. CV measurements revealed that energy band gaps calculated on the basis of ionization potential and electron affinity of the Re(I) complexes, which are correlated with energy levels of frontier molecular orbitals, fall in the range of 2.14–2.32 eV, being lower than the corresponding ligands, what makes the complexes promising for organoelectronic applications. In solution they exhibited photoluminescence quantum yields ranging from 0.15% to 84.42% depending on the chemical structure. The presence of pyrazine units significantly reduced ability for radiative emission. Most of the devices with Re(I) complexes exhibited red or orange emission, while diodes with ligands showed maximum of emission band located mainly in the green region. It should be noticed that diodes with active layer consisting of a neat complex emitted light under applied voltage.[3]
On the other hand, terpyridine ligands can form “closed-shell” octahedral 2+-tpy> complexes, which provide a linear and stable linkage in supramolecular chemistry. Numerous supramolecular architectures have been achieved using modified terpyridine ligands including Sierpiński triangles, hexagonal gasket and supramolecular rosettes[1].
References
1.Wei C, He Y, Shi X, Song Z. Terpyridine-metal complexes: Applications in catalysis and supramolecular chemistry[J]. Coordination Chemistry Reviews, 2019, 385:1-19
2.Förster C, Dorn M, Reuter T, Otto S, Davarci G, Reich T, Carrella L, Rentschler E, Heinze K. Ddpd as expanded terpyridine: Dramatic effects of symmetry and electronic properties in first row transition metal complexes[J]. Inorganics, 2018, 6(3):86
3.Klemens T, Świtlicka A, Machura B, Kula S, Krompiec S, Łaba K, Korzec M, Grzelak J, Maćkowski S. A family of solution processable ligands and their Re(I) complexes towards light emitting applications[J]. Dyes and Pigments, 2019, 163:86-101
You may like
The benefits of Milk Thistle Seed Oil
Apr 16, 2024
Abametapir:Introduction and Synthesis
Dec 25, 2023
What is 1,8-Diazabicyclo[5.4.0]undec-7-ene?
Jul 15, 2020
See also
Lastest Price from 2,2':6',2''-TERPYRIDINE manufacturers
2,2':6',2''-TERPYRIDINE
US $39.00-553.00/g2025-07-08
- CAS:
- 1148-79-4
- Min. Order:
- 5g
- Purity:
- 0.98
- Supply Ability:
- 25kg
2,2':6',2''-TERPYRIDINE
US $0.00-0.00/KG2024-08-18
- CAS:
- 1148-79-4
- Min. Order:
- 1KG
- Purity:
- 99.0%
- Supply Ability:
- 10 tons