11,12-Dihydroindolo[2,3-a]carbazole: Overview, Preparation Method and Applications in Anion Complexation and Sensing
General Description
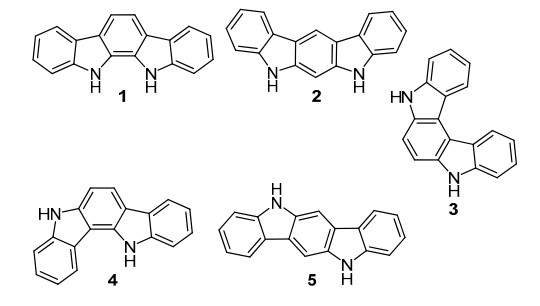
11,12-Dihydroindolo[2,3-a]carbazole is a unique compound with a fused ring structure that combines the features of indole and carbazole moieties. It holds potential for various applications in organic synthesis, pharmacology, and materials science. The compound can be efficiently prepared using o-dibromobenzene and o-phenylenediamine through C-N and C-C coupling reactions with the aid of catalysts and bases. One notable application of 11,12-Dihydroindolo[2,3-a]carbazole is in anion complexation and sensing. Alongside other synthetic derivatives, it acts as an effective anion-binding agent, demonstrating selective binding and detection of specific anions. Its structural properties allow for the formation of stable complexes with anionic species, making it a valuable tool in supramolecular chemistry. This compound and its derivatives contribute to advancements in analytical and environmental monitoring, offering innovative solutions for anion recognition and detection challenges. Overall, 11,12-Dihydroindolo[2,3-a]carbazole presents exciting opportunities for further exploration in scientific fields, with potential implications for sensor technology and chemical sensing systems.
![Figure 1. 11,12-Dihydroindolo[2,3-a]carbazole.png Article illustration](/NewsImg/2024-03-15/6384609299459605815443451.jpg)
Figure 1. 11,12-Dihydroindolo[2,3-a]carbazole
Overview
11,12-Dihydroindolo[2,3-a]carbazole is a unique organic compound belonging to the indole-carbazole family. This molecule is characterized by its intricate fused ring structure, which combines the features of both indole and carbazole moieties. Its name is derived from the specific positioning of the dihydro substituent at the 11 and 12 positions within this framework. The compound exhibits interesting properties and has the potential for various applications in fields such as organic synthesis, pharmacology, and materials science. However, further research is required to fully explore its behavior and utilization in these areas. Overall, 11,12-Dihydroindolo[2,3-a]carbazole represents a fascinating compound with significant potential for future scientific exploration. 1
Preparation Method
The preparation method of 11,12-Dihydroindolo[2,3-a]carbazole involves a series of reactions using o-dibromobenzene and o-phenylenediamine as starting compounds. This method yields a high efficiency of 92% and utilizes reagents such as sodium tert-butoxide, along with the catalysts palladium diacetate, copper bromide (CuBr), and X-Phos. The reaction takes place at room temperature, then is heated to 110°C for 24 hours. Specifically, the process involves the following steps: First, the o-dibromobenzene and o-phenylenediamine undergo C-N coupling and C-C coupling reactions under the influence of a catalyst, ligand, and base. The catalysts used are palladium salt and copper salt, where the palladium salt catalysts can include palladium chloride, palladium acetate, and others, while the copper salt catalysts encompass cuprous iodide, cuprous chloride, and related compounds. Additionally, a phosphine ligand and an organic or inorganic base are employed in the reaction. This method is advantageous due to its use of readily available raw materials and its cost-effectiveness. Furthermore, compared to previous methods, this approach requires fewer reaction steps, resulting in lower production costs and environmental impact. By utilizing cheap and easily accessible starting materials and optimizing the reaction conditions, this method demonstrates promise for the efficient and sustainable synthesis of 11,12-Dihydroindolo[2,3-a]carbazole. 1
Applications in anion complexation and sensing
11,12-Dihydroindolo[2,3-a]carbazole, along with other synthetic derivatives such as indole, biindole, and carbazole-based receptors, plays a significant role in anion complexation and sensing applications. These compounds have garnered attention as effective anion-binding agents, showcasing their potential in molecular recognition and structural chemistry for anion detection. By serving as neutral yet highly efficient anion receptors and sensors, these molecules demonstrate promising capabilities in selectively binding and detecting specific anions. Their structural properties allow for the formation of stable complexes with various anionic species, enabling them to function as molecular sensors for detecting target anions in solution. The utilization of 11,12-Dihydroindolo[2,3-a]carbazole and related compounds in anion complexation and sensing applications signifies a growing trend in the development of advanced materials for analytical and environmental monitoring purposes. Their unique chemical structures and binding properties make them valuable tools in the field of supramolecular chemistry, offering innovative solutions for anion recognition and detection challenges. Overall, 11,12-Dihydroindolo[2,3-a]carbazole present exciting opportunities for enhancing our understanding and manipulation of anion interactions, paving the way for novel advancements in sensor technology and chemical sensing systems. 2
Reference
1. Zhu YF, Pei XD, Yang XG, Wu ZK, Zhang L, Luo YH. Preparation method of indolo [2,3-a] carbazole using o-dibromobenzene and o-phenylenediamine. 2021. Patent Number: CN112250685.
2. Gale PA. Synthetic indole, carbazole, biindole and indolocarbazole-based receptors: applications in anion complexation and sensing. Chem Commun (Camb). 2008;(38):4525-4540.
Related articles And Qustion
See also
Lastest Price from 11,12-Dihydroindolo[2,3-a]carbazole manufacturers
![60511-85-5 11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE](/ProductImageEN/2022-09/Small/daecc143-0414-4491-b66f-d3880e80344d.png)
US $0.00-0.00/KG2025-04-04
- CAS:
- 60511-85-5
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
![60511-85-5 11,12-DIHYRDOINDOLO[2,3-A]CARBAZOLE](/ProductImageEN/2024-07/Small/7d4ae3eb-100f-48f0-99ae-b53724eb9a7c.jpg)
US $0.00-0.00/kg2025-03-26
- CAS:
- 60511-85-5
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20 tons
![60511-85-5 Overview of 11,12-Dihydroindolo[2,3-a]carbazole Preparation Method of 11,12-Dihydroindolo[2,3-a]carbazole Applications of 11,12-Dihydroindolo[2,3-a]carbazole in anion complexation and sensing](https://www.chemicalbook.com/CAS/GIF/60511-85-5.gif)