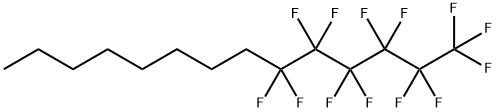
1-(Perfluorohexyl)octane: A Multifaceted Agent for Dry Eye Disease and Retinal Surgery
1-(Perfluorohexyl)octane is a prescription medicine that reduces tear evaporation and treats signs and symptoms of dry eye disease. 1-(Perfluorohexyl)octane ophthalmic solution is a single-entity, water-, steroid- and preservative-free, first-in-class semifluorinated alkane that is approved in the USA for the treatment of the signs and symptoms of dry eye disease (DED). DED is often linked with meibomian gland dysfunction (MGD), which causes an excessive evaporation of tears. 1-(Perfluorohexyl)octane ophthalmic solution stabilizes the lipid layer of the tear film and inhibits tear evaporation by forming a monolayer at the air-liquid interface. In the phase III GOBI and MOJAVE trials in adults with DED associated with MGD, one drop of 1-(Perfluorohexyl)octane ophthalmic solution instilled in each eye four times daily over 8 weeks resulted in statistically significant and clinically meaningful improvements in the signs and symptoms of DED compared with hypotonic saline (0.6%). The agent was generally well tolerated, with most ocular adverse events being mild or moderate in severity.

Efficacy of 1-(Perfluorohexyl)octane for the Treatment of Patients with Dry Eye Disease
1-(Perfluorohexyl)octane is an inert semifluorinated alkane which has emerged as a novel agent and was recently approved in Europe as a medical device for the treatment of DED. This fully saturated, linear molecule is characterized by a hydrogenated carbon chain, which confers hydrophobic properties to the compound. 1-(Perfluorohexyl)octane has multiple mechanisms of action. Primarily, it replaces the defective tear film lipid layer while forming a protective overlay at the tear film-air interface, significantly reducing evaporation rate. The structural properties of the hydrocarbon chains in meibum lipids play a crucial role in maintaining tear film stability. The degree of unsaturation of these chains determines the fluidity and spreading of the lipid layer on the ocular surface. Less unsaturated lipids are stiffer, which increases lipid clumping, eventually obstructing meibomian glands. 1-(Perfluorohexyl)octane improves the structure of the lipid layer, stabilizing the tear film and reducing DED symptoms. Finally, 1-(Perfluorohexyl)octane shows oxygen-carrying capacity, allowing the delivery of nonreactive oxygen species to the cornea, which is critical for the health of the ocular surface.[1]
In this meta-analysis, the efficacy and safety of eye drops based on semifluorinated alkane 1-(Perfluorohexyl)octane (F6H8) have been evaluated across available RCTs. These eye drops only include a lipidic component; therefore, they may be considered as the first water-free eye drops. However, unlike typical lipid-containing eye drops, which are often viscous, perfluorohexyloctane has a refractive index similar to water, minimizing vision blurring. The mechanism of action of 1-(Perfluorohexyl)octane has been extensively investigated. Its efficacy depends on the replenishment and enhancement of the lipid layer of the tear film. Both in vitro and in vivo studies demonstrated that perfluorohexyloctane eye drops reinforce the lipid layer, greatly reducing the evaporation rate of the aqueous layer of the tear film. Such effect is determined by 1-(Perfluorohexyl)octaneʼs hydrophobic structure and low surface tension, allowing for uniform distribution of the eye drops on the ocular surface, and forming a protective layer on top of the defective meibum, at the tear film-air interface. In addition, perfluorohexyloctane has oxygen-carrying capacity, which improves healing in DED patients.[2]
Additionally, the 1-(Perfluorohexyl)octane formulations used were preservative-free. This was not always the case for the control saline solutions. Except for Schmidl et al., saline solutions were either preserved with benzalkonium chloride (BAK) or the authors did not provide information about the presence of preservatives. BAK has been clearly associated with ocular surface toxicity. BAK induces an inflammatory response, alters the viability and functionality of meibomian glands, damages corneal epithelial cells, and triggers conjunctival goblet cell apoptosis. Therefore, chronic exposure to BAK may lead to tear film instability, exacerbating the symptoms of DED. In conclusion, the results of this meta-analysis demonstrated a significant effect of 1-(Perfluorohexyl)octane in reducing tCFS and eye dryness symptoms. 1-(Perfluorohexyl)octane is an effective and safe alternative for the treatment of evaporative DED; however, more well-designed non-sponsored RCTs are required to investigate the impact on TFBUT, MGD score, and ST values.
Use of 1-(Perfluorohexyl)octane as a long-term internal tamponade agent
To report the use of 1-(Perfluorohexyl)octane, a liquid semifluorinated alkane that is heavier than water, as an internal tamponade agent in surgery for complicated retinal detachments. In 23 consecutive eyes (23 patients, 19 men and four women, mean +/- standard deviation (SD) age of 58.5 years +/- 16.1) 1-(Perfluorohexyl)octane was used for long-term internal tamponade. Included were eyes with complicated retinal detachment involving the lower two quadrants of the fundus. Excluded were patients with diseases in the fellow eye or severe systemic disease. A pars plana vitrectomy was performed, including membrane peeling and retinotomy where necessary. In 23 consecutive eyes (23 patients, 19 men and four women, mean +/- standard deviation (SD) age of 58.5 years +/- 16.1) 1-(Perfluorohexyl)octane was used for long-term internal tamponade. Included were eyes with complicated retinal detachment involving the lower two quadrants of the fundus. Excluded were patients with diseases in the fellow eye or severe systemic disease. A pars plana vitrectomy was performed, including membrane peeling and retinotomy where necessary.
The mean duration for1-(Perfluorohexyl)octane being left in situ was 76 days (SD 37.64) (range, 35-202 days). Four weeks following the removal of perfluorohexyloctane 19 of the 23 patients had total reattachment of the retina; three eyes had a recurrence of retinal detachment. One patient was lost to follow-up. The mean follow-up after 1-(Perfluorohexyl)octane removal was 97 days (range, 48 to 169 days). Cataract formation or progression was noted in nine of the 10 eyes. There were two cases with high intraocular pressures. Dispersion into small droplets was observed as early as 3 days postoperatively in three of the 23 patients. At least 12 of the 23 patients had an obvious dispersion by the time of perfluorohexyloctane removal. There was no sign of optic atrophy, retinal necrosis, or retinal vascular occlusion. 1-(Perfluorohexyl)octane was tolerated as a long-term internal tamponade agent without obvious signs of damage to the retina or optic disk. Of all the complications noted, the most common was that of dispersion of the perfluorohexyloctane bubble into droplets.
Efficacy and safety of 1-(Perfluorohexyl)octane as a novel therapeutic agent
1-(Perfluorohexyl)octane (F6H8), a physically and chemically inert synthetic compound, has recently emerged as a promising candidate for the treatment of DED due to its unique properties. A systematic review that only include full-length randomized controlled studies (RCTs), reporting the effects of F6H8 in three databases, PubMed, Scopus and Web of Science, was performed according to the PRISMA statement. The search period was performed between June 1, 2023, and June 21, 2023. The Cochrane risk of bias tool was used to analyze the quality of the studies selected. A total of six RCTs were included in this systematic review. F6H8 tear substitutes treatment achieved a higher improvement than control group interventions in most of the reported variables.[3]
The mean differences between both groups were in favor of 1-(Perfluorohexyl)octane and were as follow: eye dryness score (EDS) base on a visual analogue scale (VAS) of -6.12 ± 4.3 points, ocular surface disease index (OSDI) questionnaire score of -2.8 ± 2.3 points, lipid layer thickness (LLT) of 11.4 ± 10.4 μm, total corneal fluorescein staining (tCFS) of -0.8 ± 0.3 points and ocular treatment-emergent adverse events (TEAEs) of -0.66 ± 1.7. Tear film break-up time (TBUT) was the only variable in favor of control group with a mean of -0.5 ± 0.4 s. Patient satisfaction after 1-(Perfluorohexyl)octane tear substitutes treatment was high. Therefore, F6H8 tear substitutes improve dry eye symptoms and signs with a satisfactory tolerability and could be recommended in patients with DED.
References
[1]Taloni A, Coco G, Pellegrini M, Scorcia V, Giannaccare G. Efficacy of Perfluorohexyloctane for the Treatment of Patients with Dry Eye Disease: A Meta-Analysis. Ophthalmic Res. 2025;68(1):41-51.
[2]Kirchhof B, Wong D, Van Meurs J, Hilgers RD, Macek M, Lois N, Schrage NF. Use of perfluorohexyloctane as a long-term internal tamponade agent in complicated retinal detachment surgery. Am J Ophthalmol. 2002 Jan;133(1):95-101.
[3]Ballesteros-Sánchez A, De-Hita-Cantalejo C, Sánchez-González MC, Jansone-Langine Z, de Sotomayor MA, Culig J, Sánchez-González JM. Perfluorohexyloctane in dry eye disease: A systematic review of its efficacy and safety as a novel therapeutic agent. Ocul Surf. 2023 Oct;30:254-262.
US $0.00-0.00/KG2025-12-02
- CAS:
- 133331-77-8
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS
US $0.00/KG2025-11-29
- CAS:
- 133331-77-8
- Min. Order:
- 2000KG
- Purity:
- 99.9%
- Supply Ability:
- 20tons