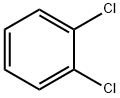
1,2-Dichlorobenzene-a hazard organic compound
1,2-Dichlorobenzene, also named orthodichlorobenzene (ODCB), is an organic compound with the formula C6H4Cl2. It is a non-polar colorless liquid that is poorly soluble in water but miscible with most organic solvents. This derivative of benzene differs from the parent compound by the presence of two adjacent chlorine atoms. 1,2-dichlorobenzene is used as a precursor for agrochemicals, as a solvent for fullerenes, as an insecticide and as an agent to remove carbon-based contamination from metal.
It is mainly used as a precursor chemical in the synthesis of agrochemicals, as a preferred solvent for dissolving and working with fullerenes, as an insecticide, and in softening and removing carbon-based contamination on metal surfaces [1].
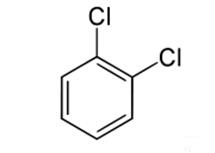
1,2-Dichlorobenzene is obtained as a side-product of the production of chlorobenzene:
C6H5Cl+ Cl2→C6H4Cl2+ HCl
The reaction also affords the 1,4- and small amounts of the 1,3-isomer. The 1,4- isomer is preferred over the 1,2- isomer due to steric hindrance. The 1,3- isomer is uncommon because it is a meta- compound, while chlorine, like all halogens, is ortho/para- directors in terms of electrophilic aromatic substitution.
1,2-Dichlorobenzene is mainly used as a precursor to 1,2-dichloro-4-nitrobenzene, an intermediate in the synthesis of agrochemicals [2]. In terms of niche applications, 1,2-dichlorobenzene is a versatile, high-boiling solvent. It is a preferred solvent for dissolving and working with fullerenes. It is an insecticide for termites and locust borers, historically used by the United States Forest Service to combat widespread bark beetle outbreaks [3]. 1,2-Dichlorobenzene is also used in softening and removing carbon-based contamination on metal surfaces.
1,2-Dichlorobenzene is toxic and bioconcentrates. Additionally, it may be considered persistent due to its lack of biodegradation where microbial communities are not acclimatised. Member countries may wish to undertake a more in-depth exposure analysis and if then indicated, a risk assessment may be considered.
Data from human exposure to 1,2-dichlorobenzene shows that concentrations of 100 ppm have been reported to cause sporadic irritation of the eyes and respiratory tract [4]. Skin irritation has been observed following dermal application in humans and animals. The Occupational Safety and Health Administration and the National Institute for Occupational Safety and Health have set occupational exposure limits at a ceiling of 50 ppm, over an eight-hour workday [5].
1,2-Dichlorobenzene is absorbed via the oral route. Absorption via the dermal or inhalation routes is poorly characterized. Inhalation is expected to be the major route for human exposure. The available toxicological data indicate that metabolic profiles and effects from 1,2-dichlorobenzene exposure are similar in rats, mice and humans. Animal studies with rats and mice have shown 1,2-dichlorobenzene to induce acute hepatotoxic effects. The LD50 for a single oral exposure to 1,2-dichlorobenzene for the rat ranges from 1516 to 2138 mg/kg bw. The LC100 for the rat is ≤ 977 ppm (5.9 mg/L) for a 10 hour exposure. During a 4-hour exposure, 1 of 20 rats died at 941 ppm (5.6 mg/L). In humans, the acute effects of 1,2-dichlorobenzene by ingestion or inhalation are reported to be headache, nausea, vomiting, vertigo, malaise and unconsciousness. Several oral studies of rats and mice ranging from 10 days to 2 years duration indicate that the adverse effects include increases in liver and kidney weights and hepatotoxicity. From these repeat dose studies, the NOAEL for non-neoplastic effects were 60 mg/kg bw, while the LOAEL was 120 mg/kg bw due to increased renal tubular regeneration in male mice.
In several microbial organisms and mammalian systems, 1,2-dichlorobenzene tested negative in vitro. However, it did induce sister chromatid exchanges in Chinese Hamster ovary cells and increased mutation frequency in mouse lymphoma cells, both in the presence of metabolic activation. 1,2-dichlorobenzene was negative in several in vivo mammalian tests, except one of two micronuclei assays in mouse bone marrow was positive. In a two-year oral study in rats and mice, 1,2-dichlorobenzene was considered not to be carcinogenic (maximum dose of 120 mg/kg bw). In an inhalation 2-generation reproduction study in rats, no fertility effects were observed and reduced pup weight during lactation occurred at doses toxic to adults. The NOAEL and LOAEL (kidney and liver effects) for adult rats were 50 (0.3 mg/L) and 150 ppm (0.6 mg/L) respectively. In developmental studies in rats and rabbits, developmental effects were only seen in rats at maternally toxic doses (400 ppm, 2.4 mg/L). No human epidemiological studies have been conducted.
1,2-Dichlorobenzene has been tested on a wide range of aquatic organisms under acute exposure, although chronic data are scarce. Results for fish ranged from 96 h LC50=1.58 mg/L for rainbow trout to 57 mg/L for fathead minnow. Both acute and chronic toxicity to aquatic invertebrates were obtained with two results showing high acute toxicity, namely EC50’s of 0.78 mg/L and 0.66 mg/L to Daphnia and Ceriodaphnia respectively. Results from exposure to algae showed EC50 values in the 1-100 mg/L range for 1,2-dichlorobenzene. Toxicity to microorganisms can be considered slight.
References
[1] https://en.wikipedia.org/wiki/1,2-Dichlorobenzene
[2] Gerald Booth (2007). "Nitro Compounds, Aromatic" in Ullmann's Encyclopedia of Industrial Chemistry Wiley-VCH, Weinheim, 2005.
[3] stensland8 (25 July 2011). "Battle of the Beetles". Retrieved 22 April 2018 – via YouTube.
[4] "CDC - Immediately Dangerous to Life or Health Concentrations (IDLH): o-Dichlorobenzene - NIOSH Publications and Products". www.cdc.gov. 16 November 2017. Retrieved 22 April 2018.
[5] "CDC - NIOSH Pocket Guide to Chemical Hazards -o-Dichlorobenzene". www.cdc.gov. Retrieved 22 April 2018.
See also
Lastest Price from 1,2-Dichlorobenzene manufacturers

US $1.00/KG2025-04-21
- CAS:
- 95-50-1
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 10 mt

US $9.00/KG2024-10-11
- CAS:
- 95-50-1
- Min. Order:
- 1KG
- Purity:
- 99.8%
- Supply Ability:
- 100tons