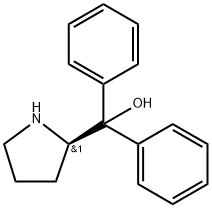
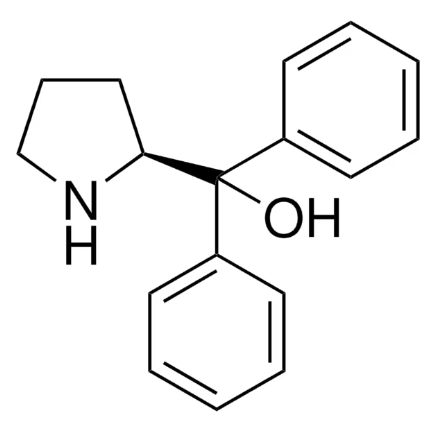
(S)-α,α-Diphenyl-2-Pyrrolidinemethanol: A Chemical Marvel
(S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol is a very important chiral β-amino alcohol catalyst precursor.
Uses
(S)-α,α-Diphenyl-2-Pyrrolidinemethanol has found numerous applications in various fields, primarily due to its chiral properties and reactivity. In the pharmaceutical industry, it serves as a valuable chiral building block for the synthesis of various drugs.
The catalyst generated in situ by reacting (S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol with borane-diethylaniline, can efficiently catalyze the enantioselective reduction of 2′-fluoroacetophenone.
Mesoporous SBA-15 silica functionalized with (S)-(-)-α,α-Diphenyl-2-pyrrolidinemethanol can catalyze the addition of diethylzinc to benzaldehyde to form (S)-1-phenyl-propanol.
Synthesis
Optically pure (S)-α,α-diphenyl-2-pyrrolidine methanol was prepared from L-proline via protection of the amino group, reaction with the Grignard reagents and deprotection of the amino-protected groups in 54.4% yield. The synthetic conditions to prepare (S)-α,α-diphenyl-2-pyrrolidinemethanol were optimised. Single crystal X-ray diffraction analysis revealed that the molecular structure of the compound was enantiomerically pure. The crystals are orthorhombic, space group P2(1)2(1)2(1).
[1] Kaufman T S, Ponzo V L, Zinczuk J. A TRIPHOSGENE-BASED SYNTHESIS OF (S)-α, α-DIPHENYL-2-PYRROLIDINEMETHANOL[J]. Organic preparations and procedures international, 1996, 28(4): 487-490.
References:
[1] T. KAUFMAN J Z V Ponzo. A TRIPHOSGENE-BASED SYNTHESIS OF (S)-α,α-DIPHENYL-2-PYRROLIDINEMETHANOL[J]. Organic Preparations and Procedures International, 1996, 28 1: 487-490. DOI:10.1080/00304949609356560.See also
Lastest Price from (R)-(+)-a,a-Diphenyl-2-pyrrolidinemethanol manufacturers

US $0.00-0.00/kg2025-09-23
- CAS:
- 22348-32-9
- Min. Order:
- 10kg
- Purity:
- 99%min
- Supply Ability:
- 1 tons

US $0.00/KG2025-04-15
- CAS:
- 22348-32-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 500000kg