Ensitrelvir: an oral antiviral drug
Introduction
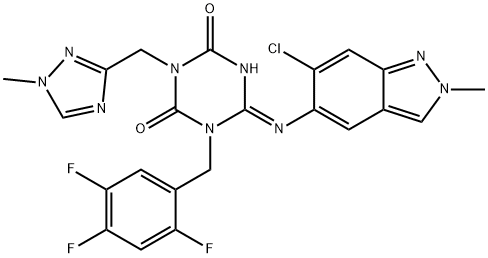
Ensitrelvir (known in Japan as Xocova®), an oral antiviral drug for COVID-19 currently approved under the emergency regulatory approval system in Japan, is a 3CL protease inhibitor created through joint research between Hokkaido University and Shionogi[1].
Mechanism of action
SARS-CoV-2 has an enzyme called 3CL protease, which is essential for the replication of the virus. Ensitrelvir suppresses the replication of SARS-CoV-2 by selectively inhibiting the 3CL protease. Ensitrelvir is the first antiviral agent to show both clinical symptomatic efficacy for five typical Omicron-related symptoms (primary endpoint) and antiviral efficacy (key secondary endpoint) in a predominantly vaccinated population of patients with mild-to-moderate SARS-CoV-2 infection, regardless of risk factors, in the results of the Phase 3 part of the Phase 2/3 study conducted during the Omicron-dominant phase of the epidemic.
Clinical research
Ensitrelvir fumaric acid is an oral SARS-CoV-2 3C-like protease inhibitor that has shown antiviral effects against multiple SARS-CoV-2 variants of concern, including Omicron subvariants, in in vitro and in vivo studies. As of September 2023, ensitrelvir (125-mg tablets) obtained emergency use approval in Japan and has been under Fast Track review by the US Food and Drug Administration (FDA). A seamless phase 2/3, double-blind, placebo-controlled randomized clinical trial is underway. Ensitrelvir treatment demonstrated decreased viral load vs placebo in phases 2a and 2b and showed improvements in 4 respiratory symptoms (stuffy or runny nose, sore throat, shortness of breath, and cough) and the composite of the 4 respiratory symptoms and feverishness in phase 2b. The SCORPIO-SR trial, phase 3 of the phase 2/3 trial, assessed the efficacy and safety of ensitrelvir in patients with mild to moderate COVID-19.
Safety
About safety, most adverse events were mild in severity, and no deaths were seen in the study. As observed in previous studies, the most common treatment-related adverse events were temporary decreases in high-density lipoprotein and increased blood triglycerides. Initial exploratory analyses from the Phase 2/3 study also indicated a potential for reduced risk of development of long COVID, and further evaluations in this regard are still ongoing[2].
Adverse events were assessed in 604 patients in the 125-mg ensitrelvir group, 599 in the 250-mg ensitrelvir group, and 605 in the placebo group. Adverse events occurred in 267 patients in the 125-mg ensitrelvir group (44.2%), 321 in the 250-mg ensitrelvir group (53.6%), and 150 in the placebo group (24.8%); treatment-related adverse events occurred in 148 patients in the 125-mg ensitrelvir group (24.5%), 217 in the 250-mg ensitrelvir group (36.2%), and 60 in the placebo group (9.9%). Most adverse events were mild, and most treatment-related adverse events were resolved without sequelae.
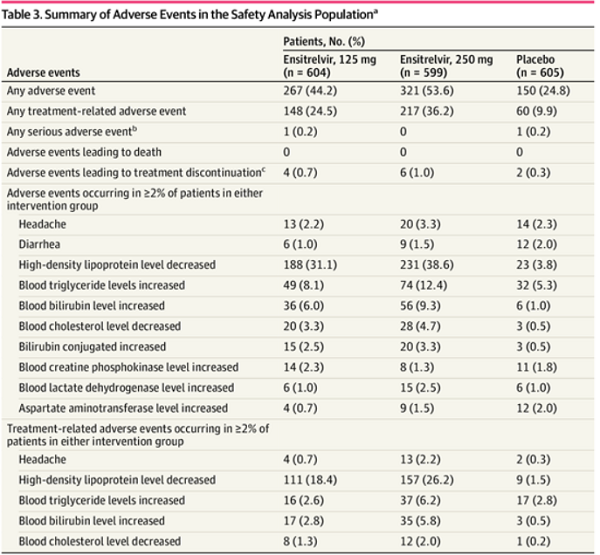
Serious adverse events were observed in 2 patients (heavy menstrual bleeding in 1 patient in the 125-mg ensitrelvir group on day 14 and acute cholecystitis in 1 patient in the placebo group on day 9), both of which were judged as not treatment-related. A decrease in high-density lipoprotein levels was the most frequently reported adverse event (188 [31.1%] in the 125-mg ensitrelvir group, 231 [38.6%] in the 250-mg ensitrelvir group, and 23 [3.8%] in the placebo group) and the most common treatment-related adverse event (111 [18.4%] in the 125-mg ensitrelvir group, 157 [26.2%] in the 250-mg ensitrelvir group, and 9 [1.5%] in the placebo group) in both ensitrelvir groups. A transient change in high-density lipoprotein cholesterol, triglyceride, total bilirubin, and iron levels was observed on day 6 in the ensitrelvir groups, which resolved without additional treatment. None of the patients developed clinical jaundice.
Adverse events leading to treatment discontinuation were recorded in 12 patients. 365 patients in the ensitrelvir groups had any treatment-related adverse events. In 335 patients (91.8%), these adverse events were resolved by day 28. No adverse events leading to death were reported.
References:
[1] TAKAHIRO TAKAZONO. Real-World Effectiveness of Ensitrelvir in Reducing Severe Outcomes in Outpatients at High Risk for COVID-19.[J]. Infectious Diseases and Therapy, 2024. DOI:10.1007/s40121-024-01010-4.[2] YOTSUYANAGI H, OHMAGARI N, DOI Y, et al. Efficacy and Safety of 5-Day Oral Ensitrelvir for Patients With Mild-to-Moderate COVID-19: The SCORPIO-SR Randomized Clinical Trial[C]. 2023. DOI:10.1101/2023.07.11.23292264.
See also

US $0.00-0.00/g2025-07-10
- CAS:
- 2647530-73-0
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 15kg
US $0.00/kg2025-07-06
- CAS:
- 2647530-73-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg