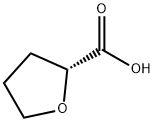
(R)-(+)-Tetrahydro-2-furoic acid: Biocatalytic Synthesis and Proton-Acceptor Properties
(R)-(+)-Tetrahydro-2-furoic acid acid is an organic which appears as a colourless to pale yellow viscous liquid, which may turn brown upon heating or prolonged storage.this kind of substance is readily soluble in water, chloroform, ethyl acetate, and similar solvents. Additionally, (R)-(+)-Tetrahydro-2-furoic acid functions as a vital raw material in fields such as biodegradable materials and coatings, demonstrating significant industrial value.

Protein engineering of an alkaline protease using (R)-(+)-Tetrahydro-2-furoic acid
Ethyl tetrahydrofuroate [(R, S)-2-ETF], as a racemic substance, has enantiomers or derivatives such as (S)-2-ethyl tetrahydrofuroate [(S)-2-ETF] and (R)-(+)-Tetrahydro-2-furoic acid, [(R)-2-TFA], which serve different functions thus can be used as raw materials in the pharmaceutical industry. These compounds are valuable as chiral building blocks in the synthesis of cephalosporins and azosines , such as terazosin and alfuzosin used for treating hypertension and gland issues. Additionally, (R)-2-TFA can act as an intermediate in synthesizing carbapenem antibiotics like ropenem. Current methods for synthesizing optically pure (S)-2-ETF and (R)-(+)-Tetrahydro-2-furoic acid typically involve acid-base salt formation and recrystallization. This process involves reacting (R, S)-2-ETF with chiral auxiliaries to form salts, separating the enantiomers based on their differences in solubility, and then synthesizing optically pure (R)-2-ETF through ethyl. Baldessari et al. observed significant regioselective behavior in lipase-catalyzed esterification and transesterification of pyridoxine with carboxylic acids or alkyl carboxylates. Yan et al. used Aspergillus oryzae lipase as a catalyst to obtain optically pure (R)-(+)-Tetrahydro-2-furoic acid methyl ester, albeit with a low yield of only 26 %.However, the protease exhibited low catalytic efficiency and poor selectivity, resulting in low yields of optically pure (S)-2-ETF and (R)-2-TFA. To achieve high enantiomeric selectivity, the active pocket of the enzyme needs to be reasonably modified using molecular techniques.[1]
This study optimized the reaction conditions for separating (R, S)-2-ETF using recombinant protease BLAPY310E and investigated the effects of reaction temperature, pH, and substrate concentration on resolution efficiency. The protease previously screened by Fujima et al. exhibited low catalytic efficiency and poor selectivity, leading to low yields of optically pure (S)-2-ETF and (R)-(+)-Tetrahydro-2-furoic acid. However, the catalytic activity and stereoselectivity of BLAPY310E towards (R, S)-2-ETF were significantly improved. This represents a substantial improvement compared to previously reported proteases. The ees of the product exceeded 99.9 %, with an eep of the product at 68.63 %. The yield of ethyl (S)-2-ETF reached 40.69 %, and the yield of (R)-(+)-Tetrahydro-2-furoic acid was 40.27 %. These yields were significantly higher than those achieved by the reported chemical resolution methods, and the process did not require toxic chiral auxiliaries. This enzymatic method thus provided a solid basis for the efficient production of optically pure (S)-2-ETF and (R)-(+)-Tetrahydro-2-furoic acid through biocatalysis. Consequently, it can now synthesize optically pure (R, S)-2-ETF enantiomers and derivatives with ideal catalytic performance and stereoselectivity under mild and sustainable reaction conditions. This progress establishes a foundation for the industrial-scale production of pure (S)-2-ETF and (R)-2-TFA.
Proton-acceptor properties of carbonyl-containing derivatives of (R)-(+)-Tetrahydro-2-furoic acid
Esters of (R)-(+)-Tetrahydro-2-furoic acid and sulfur- and nitrogen-containing analogs of these esters are an important and little-studied group of organic compounds. It is known that they are the final products of homo- lyric conversions of 2-(2'-tetrahydrofuryl)-l,3-diheterocycloalkanes. Studies of the behavior of these compounds in solutions are of practical importance, particularly studies of their proton-acceptor capability in weak-acid medium, as such data can provide an estimate of their reactivity and the possibility of compatibility with various systems. Carbonyl-containing derivatives of (R)-(+)-Tetrahydro-2-furoic acid form H-complexes with phenol in CCl4 solution. The proton-acceptor centers in the molecules of the derivatives of (R)-(+)-Tetrahydro-2-furoic acid are the oxygen atoms of the carbonyl group and the heterocycle. As the carbonyl group is removed further from the tetrahydrofuran ring, the magnitude of the energy of the H-complex becomes equal to that for tetrahydrofuran.[2]
References
[1] Xinjun Yu . (2024). Protein engineering of an alkaline protease from Bacillus licheniformis (BLAP) for efficient and specific chiral resolution of the racemic ethyl tetrahydrofuroate. Enzyme and Microbial Technology, 181, Article 110523.
[2] M. R. Skurko. (1982). Proton-acceptor properties of carbonyl-containing derivatives of 2-tetrahydrofuroic acid. Russian Chemical Bulletin, 31 9, 1796–1798
You may like
Lastest Price from (R)-(+)-Tetrahydro-2-furoic acid manufacturers
US $0.00/kg2025-06-21
- CAS:
- 87392-05-0
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1000kg

US $1.00/KG2025-04-21
- CAS:
- 87392-05-0
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 5000kg/month