Understanding the Applications and Safety Considerations of (2-Bromoethyl)benzene
General Description
(2-Bromoethyl)benzene is a chemical compound with moderate lipophilicity and limited solubility in water. It is widely used as an intermediate compound in various industries, including pharmaceuticals, fragrances, and fine chemicals. The compound is valued for its versatility in enabling the synthesis of various important derivatives. However, (2-Bromoethyl)benzene also exhibits acute toxicity, with an LD50 value of 811 mg/kg in rats. Occupational exposure to the compound can occur through dermal contact, inhalation, and ingestion, so precautions must be taken to minimize exposure. Ecologically, (2-Bromoethyl)benzene has low mobility in soil, and volatilization is expected from both moist and dry soils, with bioconcentration and adsorption to sediment being important fate processes in aquatic systems.

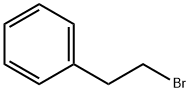
Figure 1. (2-Bromoethyl)benzene
Physical Properties
(2-Bromoethyl)benzene is a chemical compound with a molecular weight of 185.06 g/mol. It has a LogP value of 3.1, indicating moderate lipophilicity. The compound does not possess any hydrogen bond donor or acceptor sites. It has two rotatable bonds and a heavy atom count of nine. In its physical state, (2-Bromoethyl)benzene is a colorless liquid. It has a boiling point of 219 °C and a melting point of -67.5 °C. The compound is soluble in ether and benzene, while being slightly soluble in carbon tetrachloride. Its water solubility is low, with a value of 39.05 mg/l at 25 °C. The density of (2-Bromoethyl)benzene is 1.355. Regarding its vapor pressure, the compound has a value of 0.24 mmHg at 25 °C. It has a LogP value of 3.09, indicating it is more likely to dissolve in lipids than in water. (2-Bromoethyl)benzene is miscible with ether and benzene but insoluble in water. Overall, (2-Bromoethyl)benzene exhibits properties typical of an organic compound with moderate lipophilicity and limited solubility in water. 1
Versatility of (2-Bromoethyl)benzene as an Intermediate Compound
(2-Bromoethyl)benzene is widely used as an intermediate compound in various industries. It serves as a crucial starting material for the production of several beta-phenethyl derivatives, pharmaceuticals, fragrances, and other fine chemicals. In the pharmaceutical industry, (2-Bromoethyl)benzene plays a vital role in the synthesis of various medicinal compounds. It acts as a building block for the development of drugs used to treat different medical conditions. Furthermore, this compound finds applications in the fragrance industry. It is utilized in the creation of unique and appealing scents in perfumes, colognes, and other personal care products. Apart from pharmaceuticals and fragrances, (2-Bromoethyl)benzene is also employed in the production of fine chemicals. These chemicals are utilized in a wide range of applications, including specialty coatings, polymers, and additives. Overall, (2-Bromoethyl)benzene is valued for its versatility as an intermediate compound, enabling the synthesis of various important derivatives in industries such as pharmaceuticals, fragrances, and fine chemicals. 2
Toxicity and Ecological Information
(2-Bromoethyl)benzene is a chemical compound that is commonly used as a starting material in the production of various beta-phenethyl derivatives, pharmaceuticals, and fragrances. The acute toxicity of (2-bromoethyl)benzene was evaluated in male and female albino rats, which were administered a single dose of the test substance orally. The LD50 value was determined to be 811 mg/kg, and all deaths occurred within seven days of dosing. Treatment-related toxicity included hypoactivity, salivation, ataxia, clear ocular discharge, labored breathing, abnormal defecation, and hypothermia. Necropsy revealed external matting, dark red or reddened adrenal glands, stomach abnormalities, hemorrhagic thymus glands, and intestinal abnormalities. In terms of ecological information, (2-bromoethyl)benzene is expected to have low mobility in soil, and volatilization is expected from both moist and dry soils. In water, (2-bromoethyl)benzene is expected to volatilize rapidly, and bioconcentration and adsorption to sediment may be important fate processes in aquatic systems. Insufficient data are available to determine the rate or importance of biodegradation in either soil or aquatic conditions. Occupational exposure to (2-bromoethyl)benzene can occur through dermal contact, inhalation, and ingestion. As a result, precautions should be taken when handling this compound to minimize exposure. 2
Reference
1. (2-Bromoethyl)benzene. National Center for Biotechnology Information, 2023, PubChem Compound Summary for CID 7666.
2. PubChem Annotation Record for (2-BROMOETHYL)BENZENE. National Center for Biotechnology Information, 2023, Source: Hazardous Substances Data Bank.
Related articles And Qustion
Lastest Price from (2-Bromoethyl)benzene manufacturers
US $0.00-0.00/KG2025-11-28
- CAS:
- 103-63-9
- Min. Order:
- 1KG
- Purity:
- 98
- Supply Ability:
- 10000KGS

US $1.00-4.00/KG2025-09-08
- CAS:
- 103-63-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 200000KG