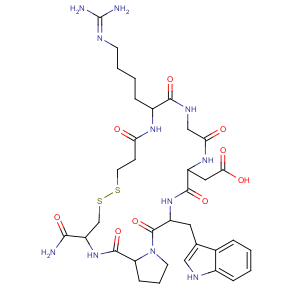
依替巴肽
Eptifibatide
148031-34-9
148031-34-9
询价
5kg
起订
10kg
起订
浙江 更新日期:2021-03-26
产品详情:
公司简介
一般项目:第二类医疗器械销售;针纺织品及原料批发;服装复试批发;日用百货销售;家用电器销售;第一类医疗器械销售;化工产品销售(不含许可类化工产品);五金产品批发;计算机软硬件及辅助设备批发;化妆品批发;消毒剂销售(不含危险化学品);专用化学产品销售(不含危验化学品);个人卫生用品销售;金属材料售;医用口罩批发;日用口罩(非医用)销售;医护人员防护用品批发;卫生用品和一次性使用医疗用品销售;劳动保护用品确售;合成材料销售;饲料添加剂销售;药物检测仪器销售;制药专用设备销售;财务咨询;信息咨询服务(不含许可类信息咨询服务);技术服务、技术开发、技术咨询、技术交流、技术转让、技术推广;染料销售;卫生用品销售;建筑装饰材料销售;电子办公设备销售;针纺识品销售;贸易经纪;销售代理;国内贸易代理(除依法须经批准的项目外,凭营业执照依法自主开展经营活动)。许可项目∶食品经营(销售预包装食品);货物进出口;药品进出口;进出口代理;技术进出口(依法须经批准的项目,经相关部门批批准方可开展经营活动,具体经营项目以审批结果为准)。
| 成立日期 | (23年) |
| 注册资本 | 1000万人民币 |
| 员工人数 | 1-10人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 贸易 |
| 主营行业 | 中间体,医药原料 |
依替巴肽相关厂家报价 更多
-

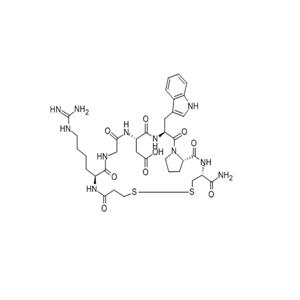
- Eptifibatide Acetate 依替巴肽
- 杭州信海医药科技有限公司 VIP
- 2026-02-14
- ¥900
-
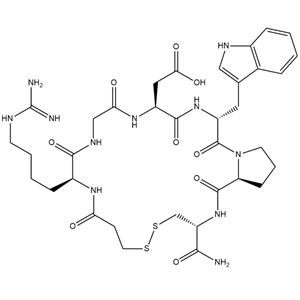
- 酸酸依非巴肽
- 浙江杰坤生物科技有限公司 VIP
- 2026-02-12
- ¥89
-
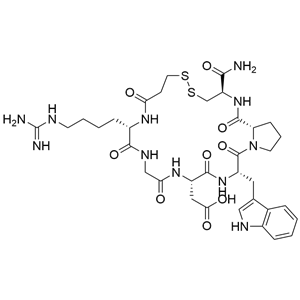
- 醋酸依非巴肽
- 杭州固拓生物科技有限公司 VIP
- 2026-02-11
- ¥50
-

- 依替巴肽-148031-34-9
- 湖北魏氏化学试剂股份有限公司 VIP
- 2026-02-10
- 询价
-

- 依替巴肽,环状七肽 | Eptifibatide
- 成都圣诺生物制药有限公司
- 2026-02-09
- 询价
-

- 醋酸依替巴肽
- 绍兴市均宇生物科技有限公司 VIP
- 2026-02-02
- ¥62
-
- 安普利肽
- 南昌探真生物技术有限公司 VIP
- 2026-01-23
- 询价
-

- 多肽合成\148031-34-9\醋酸依替巴肽Eptifibatide Acetate
- 南京源肽生物科技有限公司 VIP
- 2026-01-19
- 询价
-
- 埃替非巴肽
- 武汉裕清嘉衡药业有限公司 VIP
- 2026-01-15
- 询价
-

- 依替巴肽|T5020|TargetMol
- 上海陶术生物科技有限公司 VIP
- 2026-01-06
- ¥350