依法卡托
Ivacaftor
江苏 更新日期:2017-07-17
产品详情:
- 中文名称:
- 依法卡托
- 英文名称:
- Ivacaftor
- CAS号:
- 873054-44-5
- 品牌:
- 需询单
- 产地:
- 江苏
- 保存条件:
- 常温
- 纯度规格:
- 99
公司简介
苏州苏旺森生物医药科技有限公司成立于2016年10月,总部位于经济发达、交通便利、环境优美的独墅湖生物纳米园内。建有化学合成实验室二百余平方米,有多年有机合成经验团队数十余人,并在江西建有数千平方米工厂,该工厂通过GMP认证,并配备有50L-16000L反应釜及配套设备若干,可满足公斤级至吨位级产品的定制加工生产。
研发方面,公司以海归博士为主要研发核心,致力于催化技术开发和应用,并以此为基础展开医药高级中间体(尤其是手性中间体)、原料药、天然植物提取物的结构改造研发、生产和销售。主要适应症领域为抗肿瘤、心血管、糖尿病和抗病毒。
公司的经营团队由从业多年的专业人士组成,拥有丰富的研发经验,在国内外同多家一线药企
| 成立日期 | (10年) |
| 注册资本 | 500万 |
| 员工人数 | 10-50人 |
| 年营业额 | ¥ 1000万-5000万 |
| 经营模式 | 贸易,工厂 |
| 主营行业 | 中间体,天然产物,生物化工,医药原料 |
依法卡托相关厂家报价 更多
-

- 依伐卡托 873054-44-5 含量98% 湖北威德利 陈明 13339985473同微
- 湖北威德利化学试剂有限公司 VIP
- 2026-02-27
- 询价
-

- 依伐卡托 873054-44-5 |化学试剂|图谱|检测方法|维斯尔曼生物-王明
- 武汉维斯尔曼生物工程有限公司 VIP
- 2026-02-27
- 询价
-

- 依伐卡托
- 沧州恩科医药科技有限公司 VIP
- 2026-02-27
- ¥999
-
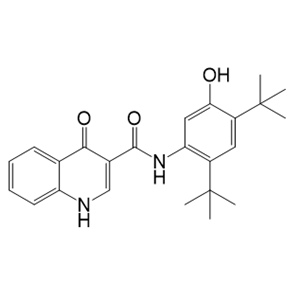
- 依伐卡托 873054-44-5
- 上海法默生物科技有限公司 VIP
- 2026-02-27
- 询价
-

- DM 873054-44-5
- 石家庄鼎旻医药科技有限公司 VIP
- 2026-02-27
- 询价
-

- 依伐卡托;873054-44-5
- 普善实业(陕西)有限公司 VIP
- 2026-02-27
- 询价
-

- 依伐卡托 873054-44-5
- 沧州恩科医药科技有限公司 VIP
- 2026-02-27
- ¥6000
-

- 依伐卡托
- 武汉福鑫化工有限公司 VIP
- 2026-02-27
- 询价
-

- 依伐卡托|T2588|TargetMol
- 上海陶术生物科技有限公司 VIP
- 2026-02-25
- ¥197
-
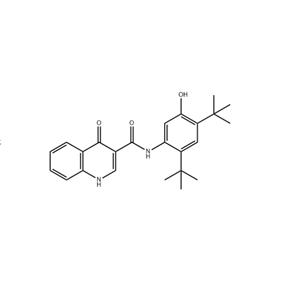
- 依伐卡托
- 济南健丰化工有限公司 VIP
- 2026-02-24
- 询价


