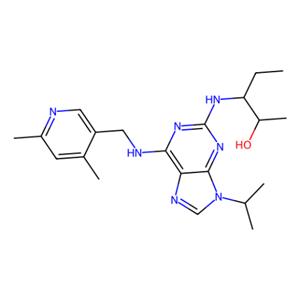
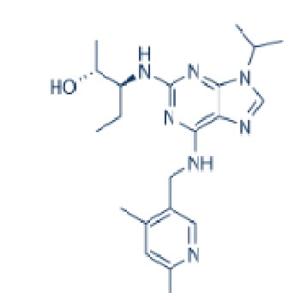
| 名称 | Fadraciclib |
| 描述 | Fadraciclib (CYC065) is an orally available, second-generation ATP-competitive inhibitor of CDK2/CDK9 kinases (IC50s: 5/26 nM). |
| 细胞实验 | Briefly, tumour cells are plated in six-well plates and treated with a titration of CYC065 concentrations (i.e., ranging from 100 to 500?nM). After 72?h, cells are harvested, washed and stained with propidium iodide (PI; 5?μg/mL) for flow cytometric counts. The percentage of viable cells is then normalized considering the vehicle-treated cells as 100% viable. Half-maximal inhibitory concentration values are determined using GraphPad Prism5 version 6. For drug combination studies, USC-ARK-1 and USC-ARK-2 cell lines are incubated with the combination of Taselisib and CYC065 at multiple paired concentrations including the IC50, the IC50/2 and the IC50*2 of each cell line to the corresponding drug (i.e., 10?nM of Taselisib and 198?nM of CYC065 for USC-ARK-1 and 50?nM of Taselisib and 62.5?nM of CYC065 for USC-ARK-2). Synergism is assessed by the combination index (CI). CI values <1 define a synergistic activity of the combination treatment. The CI values are calculated using the CompuSyn software [1]. |
| 动物实验 | Briefly, 5-7-week-old SCID mice are injected into the subcutaneous region with USC cells. A minimum of five animals per group are used. Treatments are administrated by oral gavage starting 1 week after tumor implantation when the size of the tumor is 0.125-0.150?cm3. Uterine serous carcinoma-ARK-2-derived xenografts are divided into two groups: one group of animal receive the vehicle, whereas the experimental group receives CYC065 (22.5?mg/kg daily for 3 weeks). Uterine serous carcinoma-ARK-1-derived xenografts are instead divided into four groups: one group receive the vehicle (0.5% methylcellulose-0.2% Tween-80), one group receive CYC065 (22.5?mg/kg daily for 3 weeks), one group receive Taselisib (10 mg/kg daily, 5 days per week per 3 weeks) and the last group receive the combination of CYC065 and Taselisib. The size of the tumor at the initiation of treatment is 0.125-0.150?cm3. Mouse weight and tumor size is recorded two times a week for the entire experimental period. Tumor volume is calculated. |
| 体外活性 | CYC065通过阻碍细胞在细胞周期G1阶段的活动,并特异性抑制在环形蛋白E1 (CCNE1) 过表达的子宫浆液性癌症(USCs)中的细胞生长。与低CCNE1表达的细胞系相比,高CCNE1 mRNA及蛋白水平的USC细胞系对CYC065的治疗表现出更高的敏感性(IC50: 平均±标准差=124.1±57.8nM 对CCNE1过表达的USC细胞系 vs 415±117.5nM 对CCNE1低表达者;P=0.0003)。重要的是,低浓度的CYC065(即,100nM)仅在CCNE1过表达的USC细胞系中引起细胞周期G1阶段的停滞[1]。 |
| 体内活性 | USC-ARK-2衍生的异种移植物每日使用CYC065 (22.5 mg/kg)治疗共3周。肿瘤大小与小鼠体重每周记录两次。CYC065的日常给药显著减缓了与对照组相比的肿瘤生长(治疗第9天起,P=0.012)。整个治疗期间未报告有显著的体重减轻[1]。 |
| 存储条件 | Pure form: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| 溶解度 | DMSO : 100 mg/mL (251.56 mM), Sonication is recommended.
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 4 mg/mL (10.06 mM), Sonication is recommended.
|
| 关键字 | Inhibitor | inhibit | Fadraciclib | Cyclin dependent kinase | CYC-065 | CYC 065 | CDK9 | CDK2 | CDK |
| 相关产品 | Seliciclib | PF07104091 | 2-Chloropyrazine | Sodium Oxamate | Abemaciclib methanesulfonate | Ribociclib | Abemaciclib | Amantadine | Palbociclib | GW 441756 | Dinaciclib | Kojic acid |
| 相关库 | 抑制剂库 | 经典已知活性库 | 抗癌活性化合物库 | 已知活性化合物库 | 细胞周期化合物库 | 激酶抑制剂库 | 抗衰老化合物库 | NO PAINS 化合物库 | 临床期小分子药物库 | 药物功能重定位化合物库 | 抗癌临床化合物库 | 抗癌药物库 |