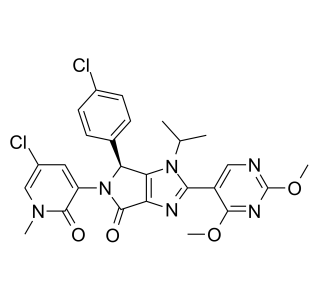
1448867-41-1
NVP-HDM201
1448867-41-1
1448867-41-1
询价
100G
起订
1G
起订
5G
起订
上海 更新日期:2026-02-12
产品详情:
公司简介
上海传芊化工科技中心是专业从事化学定制合成、抑制剂、医药中间体的高新科技技术企业。 高品质,低价格,优技术! 拥有自己的实验室,现有大量现货并可以提供定制。
| 成立日期 | (6年) |
| 注册资本 | 0 |
| 员工人数 | 1-10人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 贸易,工厂,定制,服务 |
| 主营行业 | 有机合成试剂 |
NVP-HDM201相关厂家报价
-
- 寡肽-41/Oligopeptide-41
- 南京肽研生物科技有限公司 VIP
- 2026-02-20
- ¥800
-

- 三肽-41
- 绍兴市均宇生物科技有限公司 VIP
- 2026-03-02
- ¥89
-

- 108-41-8
- 河南阿尔法化工有限公司 VIP
- 2026-03-06
- 询价
-

- 化合物 NVP-HDM 201|T5555|TargetMol
- TargetMol中国(陶术生物) VIP
- 2025-11-17
- ¥638