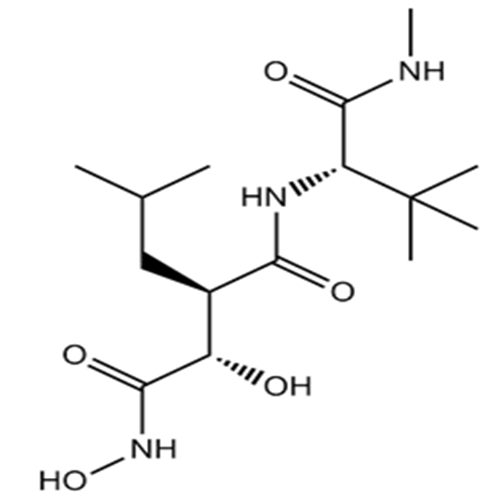
154039-60-8Marimastat
Marimastat
154039-60-8
154039-60-8
询价
1盒
起订
上海 更新日期:2025-11-06
产品详情:
- 中文名称:
- Marimastat
- 英文名称:
- Marimastat
- CAS号:
- 154039-60-8
- 品牌:
- 一研
- 保存条件:
- Store at -20°C
- 产品类别:
- 抑制剂
- Cas:
- 154039-60-8
公司简介
上海一研生物科技有限公司Shanghai yiyan bio-technology Co. Ltd.主要从事免疫学、分子生物学和常规生化试剂等为一体的科研产品销售企业,公司自成立以来,秉承""全心全意服务于科研工作者""的企业理念,立足生物科技领域,运用生物技术和科研试剂,发展现代生物科技,为各类大中小医院及其它医疗机构、高等院校、科研院所、企事业单位提供优质的产品,服务生物科技领域的科学研究人员。
公司具有对普通货物、冷藏及冷冻仓库的存储、包装及运输能力。
公司将始终坚持信誉立业、以人为本、质量保证、诚信服务的宗旨,不断拼搏,开拓进取,与各界朋友携手共创美好未来。
| 成立日期 | (12年) |
| 注册资本 | 100 |
| 员工人数 | 50-100人 |
| 年营业额 | ¥ 100万以内 |
| 经营模式 | 工厂,试剂 |
| 主营行业 | 生化试剂,抗体,细胞培养,分子生物学,免疫安全 |
Marimastat相关厂家报价 更多
-

- MMPs抑制剂(Marimastat)
- 上海泽叶生物科技有限公司 VIP
- 2026-03-10
- 询价
-

- 马立马司他 | 154039-60-8 含量98% 高纯试剂 陈明 13339985473同微
- 湖北威德利化学试剂有限公司 VIP
- 2026-03-05
- 询价
-

- 马立马司他—154039-60-8
- 湖北魏氏化学试剂股份有限公司 VIP
- 2026-02-24
- 询价
-

- 马立马司他|T6885|TargetMol
- TargetMol中国(陶术生物) VIP
- 2025-11-17
- ¥397
-
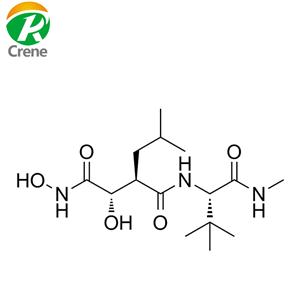
- 马立马司他
- 台州市科瑞生物技术有限公司 VIP
- 2025-07-31
- 询价
-
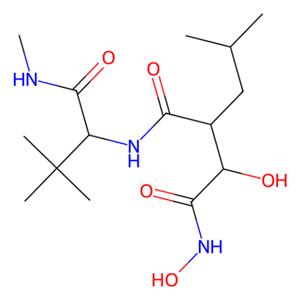
- aladdin 阿拉丁 M302985 马立马司他 154039-60-8 99%
- 上海阿拉丁生化科技股份有限公司 VIP
- 2025-05-16
- ¥448.90
-
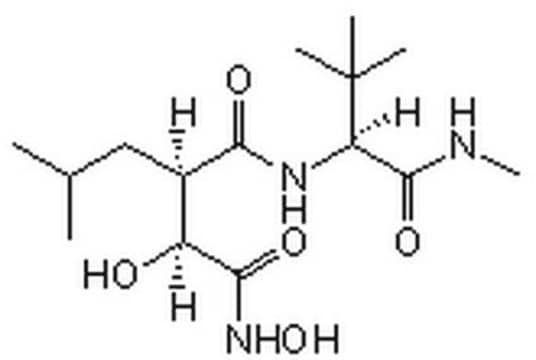
- Marimastat Calbiochem,154039-60-8
- 西格玛奥德里奇(上海)贸易有限公司 VIP
- 2023-10-27
- ¥3239.61
-

- 马立马司他
- 上海赛默罗生物科技有限公司
- 2019-11-08
- 询价
-

- Marimastat
- 上海瀚香生物科技有限公司
- 2019-11-08
- 询价