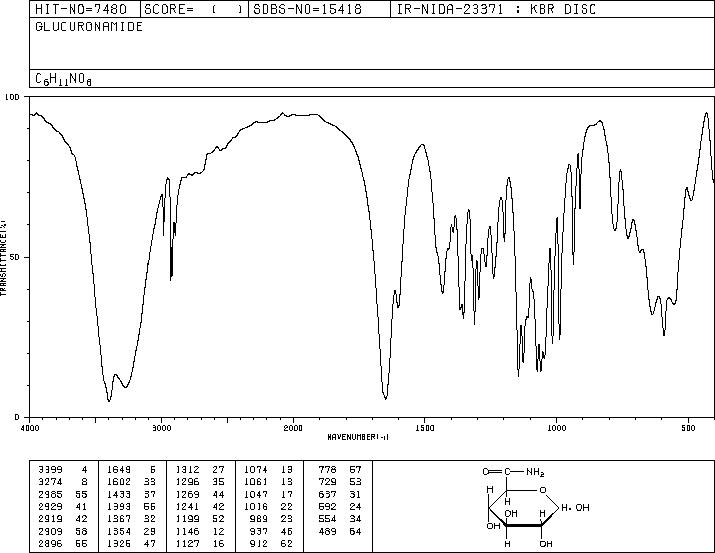
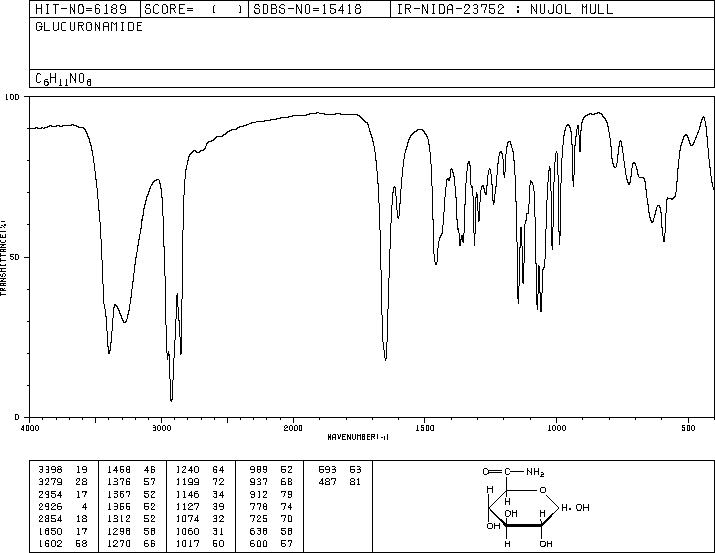
-
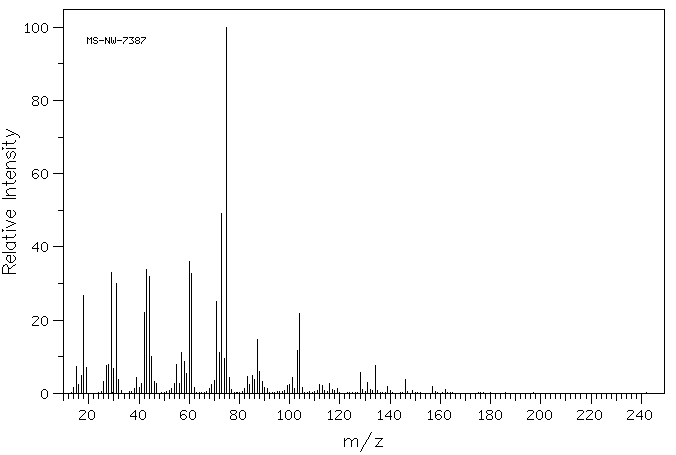
MS-NW-7387 glucuronamide C6H11NO6 (Mass of molecular ion: 193)
Source Temperature: 200 °C Sample Temperature: 180 °C Direct, 75 eV
14.0 1.6 15.0 7.4 16.0 2.3 17.0 5.0 18.0 26.7 19.0 7.0 26.0 3.2 27.0 7.7 28.0 7.9 29.0 33.0 30.0 6.9 31.0 29.9 32.0 3.8 38.0 1.3 39.0 4.2 40.0 1.6 41.0 2.7 42.0 22.0 43.0 33.8 44.0 31.8 45.0 10.2 46.0 3.2 47.0 2.7 53.0 1.4 54.0 2.7 55.0 7.8 56.0 2.7 57.0 11.2 58.0 8.8 59.0 5.5 60.0 35.9 61.0 32.8 62.0 1.7 68.0 1.3 69.0 2.3 70.0 3.4 71.0 25.2 72.0 11.2 73.0 49.1 74.0 9.5 75.0 100.0 76.0 4.4 77.0 1.0 82.0 1.2 83.0 4.6 84.0 2.4 85.0 4.8 86.0 3.8 87.0 14.7 88.0 6.0 89.0 3.2 90.0 1.6 91.0 1.3 99.0 2.1 100.0 2.5 101.0 4.4 102.0 1.3 103.0 11.7 104.0 21.8 105.0 1.7 112.0 2.3 113.0 2.1 116.0 2.7 117.0 1.1 119.0 1.2 128.0 5.6 129.0 1.0 131.0 3.0 132.0 1.0 134.0 7.6 139.0 2.0 146.0 3.9 157.0 1.9 162.0 1.1
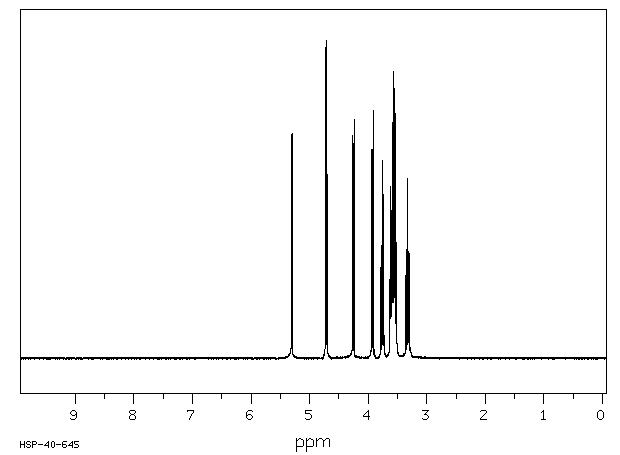
400 MHz in D2O
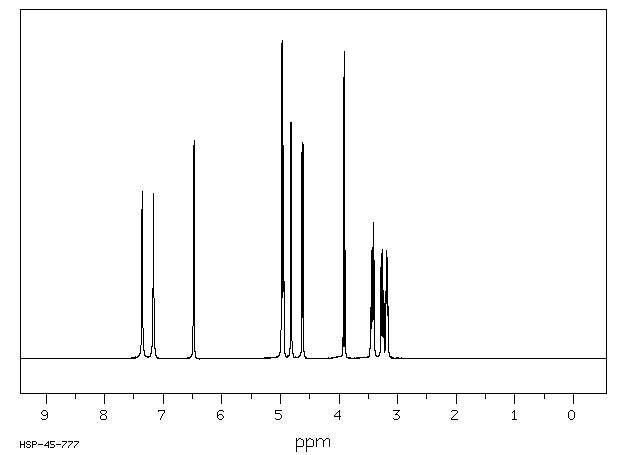
400 MHz in DMSO-d6
-
1H NMR 399.65 MHz C6 H11 N O6 saturated in D2O glucuronamide 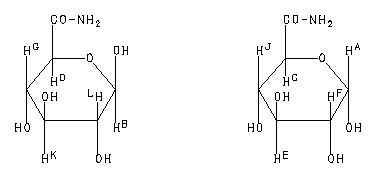
Assign. Shift(ppm)
A 5.298 B 4.713 C 4.251 D 3.926 E 3.756 F 3.608 G 3.576 J 3.551 K 3.546 L 3.323 mixture of anomers. J(A,F)=3.7Hz J(B,L)=8.1Hz J(C,J)=10.0Hz J(D,G)=9.5Hz J(E,F)=9.9Hz J(E,J)=9.8Hz J(G,K)=9.2Hz J(K,L)=8.1Hz assigned by H-H COSY.
Hz ppm Int.2124.88 5.317 30 2119.38 5.304 706 2115.72 5.294 709 1892.21 4.735 32 1891.48 4.733 36 1887.57 4.724 979 1883.18 4.713 50 1882.81 4.712 48 1879.52 4.703 1000 1704.35 4.265 703 1694.34 4.240 751 1574.22 3.939 658 1573.00 3.936 436 1564.70 3.916 782 1512.45 3.785 287 1506.59 3.770 32 1502.69 3.761 622 1493.41 3.737 515 1448.61 3.625 536 1444.82 3.616 540 1441.16 3.607 309 1438.72 3.600 465 1435.06 3.591 456 1432.01 3.584 742 1428.10 3.574 573 1424.19 3.564 798 1422.85 3.561 904 1418.21 3.549 705 1415.16 3.541 851 1411.50 3.532 133 1409.06 3.526 457 1406.13 3.519 364 1400.15 3.504 37 1399.41 3.502 34 1399.05 3.501 32 1344.85 3.366 32 1344.48 3.365 31 1342.65 3.360 261 1340.21 3.354 42 1338.62 3.350 31 1336.18 3.344 344 1335.08 3.341 280 1328.25 3.324 440 1327.03 3.321 566 1320.31 3.304 135 1318.97 3.301 330 1316.65 3.295 96 1315.06 3.291 68 1312.01 3.283 31 1311.65 3.282 31 1307.13 3.271 33
-
1H NMR 399.65 MHz C6 H11 N O6 0.038 g : 0.5 ml DMSO-d6 alpha-D-glucuronamide 
Assign. Shift(ppm)
A *1 7.36 B *1 7.17 C 6.48 D 4.98 E 4.950 F 4.82 G 4.62 J 3.910 K 3.429 L 3.261 M 3.181 J(C,E)=4.5 Hz J(D,L)=4.3 Hz J(E,M)=4.0 Hz J(F,K)=4.9 Hz J(G,M)=6.6 Hz J(J,L)=10.0 Hz J(K,M)=J(K,L)=9.8 Hz assigned by H-H and C-H COSY, and addition of D2O.
Hz ppm Int.2941.04 7.360 526 2864.14 7.167 520 2591.80 6.486 671 2587.28 6.474 688 1990.72 4.982 991 1986.45 4.971 1000 1982.42 4.961 466 1978.39 4.951 671 1974.24 4.940 438 1927.12 4.823 742 1922.24 4.810 741 1850.22 4.630 666 1843.63 4.614 680 1567.99 3.924 883 1558.11 3.899 965 1384.16 3.464 159 1379.27 3.452 169 1375.00 3.441 339 1370.24 3.429 350 1365.84 3.418 264 1314.09 3.289 285 1309.81 3.278 281 1305.18 3.266 320 1304.32 3.264 342 1300.90 3.256 336 1300.05 3.253 326 1295.41 3.242 211 1291.14 3.231 213 1280.64 3.205 197 1277.10 3.196 215 1274.17 3.189 231 1270.87 3.180 341 1267.70 3.173 205 1264.89 3.165 193 1261.23 3.156 164
更多供应商