-
MS-NW-8791 S-triazolo(4,3-a)quinoline C10H7N3 (Mass of molecular ion: 169)
Source Temperature: 190 °C Sample Temperature: 110 °C Direct, 75 eV
36.0 1.4 38.0 1.2 39.0 2.0 50.0 1.4 51.0 2.0 57.5 1.6 62.0 2.5 63.0 3.5 64.0 1.8 70.5 1.4 71.0 1.9 75.0 1.6 84.5 3.3 87.0 1.4 88.0 5.3 89.0 3.0 90.0 1.4 113.0 1.2 114.0 8.0 115.0 38.1 116.0 3.9 128.0 1.4 140.0 1.2 141.0 1.6 142.0 28.4 143.0 3.0 169.0 100.0 170.0 12.4
parameter in DMSO-d6
400 MHz in DMSO-d6
parameter in CDCl3
-
1H NMR 300 MHz C10 H7 N3 5 wt% in DMSO-d6 S-triazolo(4,3-a)quinoline 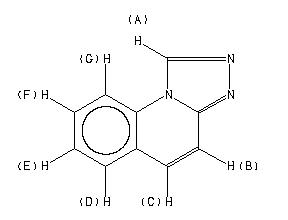
Parameter ppm Hz
D(A) 9.985 D(B) 7.729 D(C) 7.809 D(D) 8.013 D(E) 7.611 D(F) 7.788 D(G) 8.444 J(A,B) 0.56 J(A,C) 0.0 J(A,D) 0.0 J(A,E) 0.0 J(A,F) 0.0 J(A,G) 0.0 J(B,C) 9.7 J(B,D) 0.3 J(B,E) 0.0 J(B,F) 0.3 J(B,G) 0.0 J(C,D) -0.3 J(C,E) 0.0 J(C,F) 0.0 J(C,G) 0.60 J(D,E) 7.88 J(D,F) 1.44 J(D,G) 0.41 J(E,F) 7.37 J(E,G) 1.08 J(F,G) 8.31 HEFFERNAN,M.L. & IRVINE,G.M. AUST.J.CHEM. 29, 815 (1976)
Hz ppm Int.2995.52 9.985 1000 2537.41 8.458 321 2529.14 8.430 350 2408.63 8.029 274 2407.30 8.024 281 2400.80 8.003 312 2399.44 7.998 321 2348.51 7.828 308 2345.12 7.817 169 2343.67 7.812 167 2338.73 7.796 723 2336.58 7.789 333 2329.47 7.765 199 2328.02 7.760 189 2322.63 7.742 667 2312.92 7.710 305 2291.04 7.637 232 2290.00 7.633 234 2283.25 7.611 334 2282.57 7.609 326 2275.85 7.586 158 2274.81 7.583 155
-
1H NMR 399.65 MHz C10 H7 N3 0.039 g : 0.5 ml DMSO-d6 S-triazolo(4,3-a)quinoline 
Assign. Shift(ppm)
A 10.052 B 8.463 C 8.024 D 7.838 E 7.804 F 7.767 G 7.626
Hz ppm Int.4017.64 10.053 997 4016.88 10.051 1000 3386.84 8.475 394 3378.45 8.454 417 3211.82 8.037 332 3210.60 8.034 341 3203.89 8.017 365 3202.67 8.014 365 3138.73 7.854 391 3129.27 7.831 754 3126.37 7.823 233 3120.42 7.808 307 3119.20 7.805 412 3112.18 7.788 266 3110.66 7.784 270 3108.06 7.777 640 3107.45 7.776 643 3103.03 7.765 30 3097.84 7.752 358 3055.57 7.646 293 3054.50 7.643 309 3047.64 7.626 414 3043.82 7.617 40 3040.47 7.608 225 3039.40 7.606 221
-
1H NMR 300 MHz C10 H7 N3 5 wt% in CDCl3 S-triazolo(4,3-a)quinoline 
Parameter ppm Hz
D(A) 9.282 D(B) 7.652 D(C) 7.561 D(D) 7.818 D(E) 7.542 D(F) 7.692 D(G) 7.991 J(A,B) 0.6 J(A,C) 0.0 J(A,D) 0.0 J(A,E) 0.0 J(A,F) 0.0 J(A,G) 0.0 J(B,C) 9.6 J(B,D) 0.3 J(B,E) 0.0 J(B,F) 0.3 J(B,G) 0.0 J(C,D) -0.3 J(C,E) 0.0 J(C,F) 0.0 J(C,G) 0.56 J(D,E) 7.93 J(D,F) 1.44 J(D,G) 0.51 J(E,F) 7.40 J(E,G) 1.06 J(F,G) 8.36 HEFFERNAN,M.L. & IRVINE,G.M. AUST.J.CHEM. 29, 815 (1976)
Hz ppm Int.2784.83 9.283 999 2784.38 9.281 1000 2402.15 8.007 282 2401.68 8.006 300 2401.16 8.004 284 2393.85 7.979 336 2393.34 7.978 357 2392.85 7.976 341 2350.23 7.834 249 2348.87 7.830 261 2342.38 7.808 301 2340.98 7.803 322 2316.29 7.721 171 2314.82 7.716 168 2308.86 7.696 265 2307.53 7.692 285 2306.62 7.689 181 2300.84 7.669 446 2299.14 7.664 203 2291.63 7.639 651 2272.29 7.574 704 2270.28 7.568 311 2269.20 7.564 287 2262.53 7.542 657 2255.04 7.517 168 2253.94 7.513 162









![S-三唑并[4,3-a]喹啉](https://img.chemicalbook.com/SupplyImg/2021-06-23/Large/202106230548392613562.png)