-
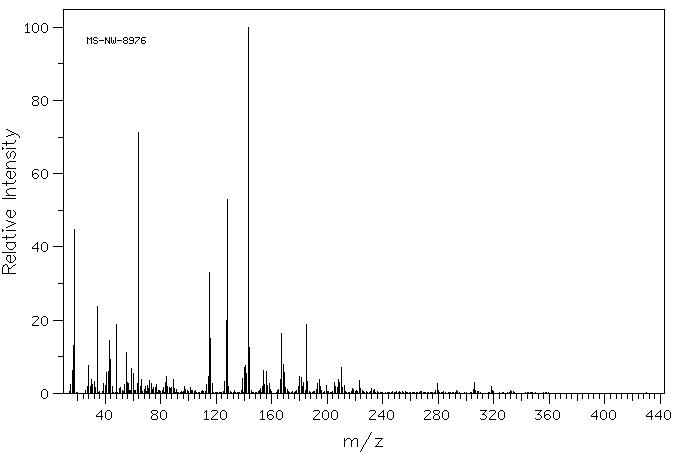
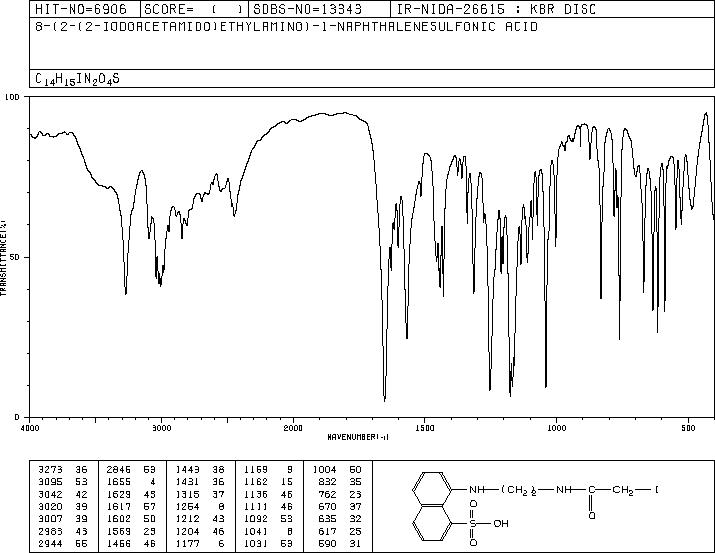
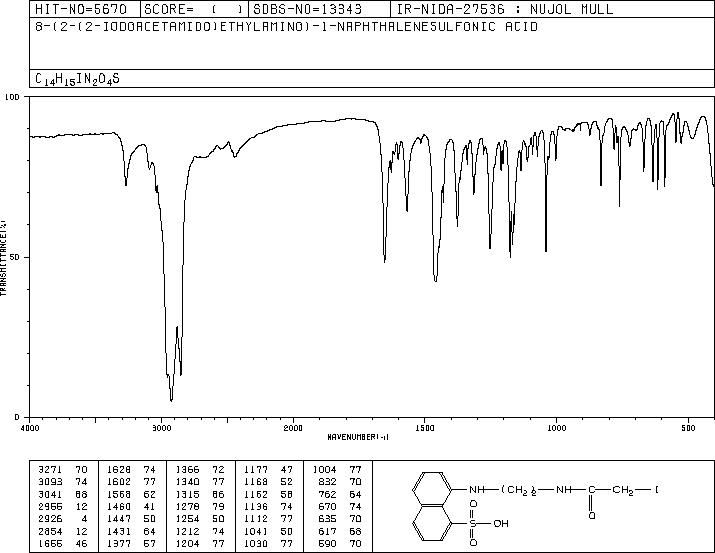
MS-NW-8976 8-(2-(2-iodoacetamido)ethylamino)-1-naphthalenesulfonic acid C14H15IN2O4S (Mass of molecular ion: 434)
Source Temperature: 220 °C Sample Temperature: 300 °C Direct, 75 eV
15.0 2.5 16.0 6.3 17.0 13.1 18.0 44.8 27.0 1.9 28.0 7.6 29.0 2.0 30.0 3.7 31.0 2.4 32.0 3.2 33.0 1.6 34.0 23.6 39.0 2.7 40.0 2.1 41.0 5.7 42.0 5.9 43.0 14.4 44.0 9.3 45.0 1.9 48.0 18.7 50.0 1.3 51.0 1.5 54.0 2.3 55.0 11.2 56.0 3.0 57.0 2.8 58.5 1.3 59.0 6.9 60.0 5.5 63.0 2.7 64.0 71.2 65.0 1.9 66.0 3.8 68.0 1.0 69.0 1.9 70.0 1.7 70.5 2.1 71.0 1.3 71.5 3.4 73.0 2.6 74.0 1.1 75.0 1.6 76.0 1.5 77.0 2.3 82.0 1.6 83.0 2.9 83.5 1.4 84.0 4.5 85.0 2.0 86.0 1.6 87.0 1.3 88.0 1.5 89.0 3.7 90.0 1.4 91.0 1.1 97.0 1.8 98.0 1.1 101.0 1.5 113.0 2.4 114.0 4.7 115.0 33.1 116.0 15.1 117.0 2.6 126.0 3.3 127.0 20.0 128.0 52.9 129.0 2.0 139.0 4.1 140.0 7.0 141.0 7.5 142.0 5.5 143.0 100.0 144.0 12.5 152.0 1.4 153.0 2.0 154.0 6.2 155.0 2.4 156.0 5.9 157.0 2.1 158.0 2.8 159.0 1.1 165.0 1.0 166.0 3.8 167.0 16.4 168.0 7.8 169.0 5.7 170.0 1.6 171.0 1.0 179.0 1.8 180.0 4.6 181.0 4.4 182.0 2.0 183.0 2.9 185.0 18.9 186.0 3.2 192.0 1.0 193.0 2.8 194.0 3.9 195.0 2.0 199.0 2.1 205.0 2.9 206.0 1.8 207.0 1.7 208.0 3.8 209.0 3.1 210.0 7.0 211.0 1.7 212.0 2.1 218.0 1.4 219.0 1.1 223.0 3.6 224.0 1.3 232.0 1.2 234.0 1.0 279.0 2.6 305.0 1.0 306.0 2.9 318.0 2.0
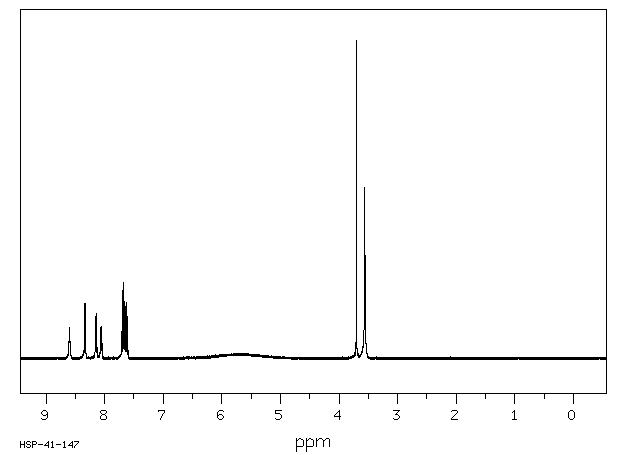
400 MHz in DMSO-d6
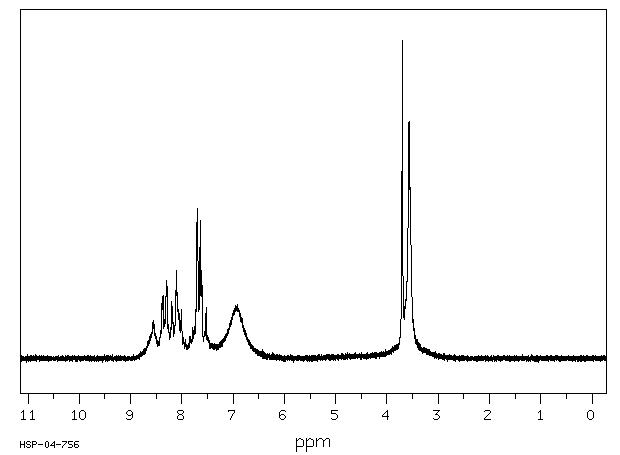
90 MHz in DMSO-d6
-
1H NMR 399.65 MHz C14 H15 I N2 O4 S ca 1 mg : 0.5 ml DMSO-d6 8-(2-(2-iodoacetamido)ethylamino)-1-naphthalenesulfonic acid 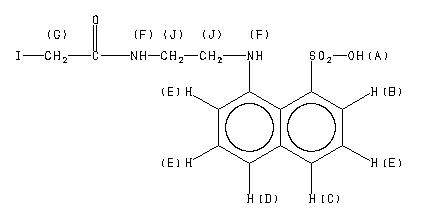
Assign. Shift(ppm)
A 8.60 B 8.337 C 8.146 D 8.060 E 7.69 F 7.66 G 7.63 K 3.700 L 3.56
Hz ppm Int.3436.52 8.599 96 3336.55 8.349 156 3335.08 8.346 174 3329.10 8.331 173 3327.76 8.327 173 3260.25 8.158 124 3259.16 8.156 133 3251.95 8.137 142 3250.85 8.135 130 3225.71 8.072 96 3224.12 8.068 98 3218.38 8.053 99 3218.02 8.053 100 3216.31 8.048 101 3081.79 7.712 85 3077.64 7.701 30 3077.15 7.700 31 3074.22 7.693 213 3071.53 7.686 36 3071.17 7.685 34 3070.56 7.684 39 3070.31 7.683 39 3069.82 7.682 42 3066.65 7.674 240 3065.55 7.671 185 3063.60 7.666 168 3060.67 7.659 34 3057.86 7.652 67 3054.57 7.644 164 3047.12 7.625 175 3046.51 7.623 177 3039.06 7.605 132 1484.13 3.714 30 1478.88 3.701 1000 1434.81 3.591 36 1434.45 3.590 37 1433.96 3.589 37 1433.35 3.587 39 1432.74 3.585 42 1432.50 3.585 42 1431.88 3.583 46 1431.52 3.582 49 1430.54 3.580 54 1430.18 3.579 57 1429.57 3.578 61 1423.10 3.561 540 1417.48 3.547 134 1416.63 3.545 138 1412.35 3.534 37 1411.87 3.533 37 1411.50 3.532 36 1411.13 3.531 36 1410.52 3.530 33 1409.79 3.528 30
-
1H NMR 89.56 MHz C14 H15 I N2 O4 S 0.033 g : 0.5 ml CDCl3 8-(2-(2-iodoacetamido)ethylamino)-1-naphthalenesulfonic acid 
Assign. Shift(ppm)
A *1 8.55 B 8.33 C 8.13 D 8.07 E 7.88 to 7.45 F *1 6.94 G 3.697 J 3.57
Hz ppm Int.766.00 8.553 120 755.06 8.431 60 754.38 8.424 76 753.88 8.418 80 753.50 8.414 86 753.00 8.408 87 750.88 8.385 190 749.25 8.366 198 748.00 8.352 109 747.63 8.348 110 747.00 8.341 116 746.56 8.336 123 745.81 8.328 124 745.19 8.321 134 743.50 8.302 247 742.13 8.287 228 740.44 8.268 107 739.81 8.261 102 739.31 8.255 102 738.00 8.241 71 736.75 8.227 77 735.88 8.217 98 735.25 8.210 107 733.81 8.194 178 732.81 8.183 149 732.50 8.179 160 730.31 8.155 85 729.94 8.151 84 729.56 8.147 88 725.75 8.104 275 724.19 8.087 209 722.50 8.068 152 720.81 8.049 143 719.31 8.032 92 717.25 8.009 154 716.94 8.006 154 714.31 7.976 58 713.81 7.971 62 713.50 7.967 65 713.13 7.963 64 710.50 7.934 58 702.56 7.845 69 702.13 7.840 70 701.50 7.833 64 701.13 7.829 60 700.75 7.825 58 700.38 7.821 59 699.31 7.809 59 697.19 7.785 97 696.19 7.774 72 695.81 7.770 73 694.63 7.757 101 693.81 7.747 93 689.63 7.701 472 686.31 7.664 160 684.06 7.639 434 681.94 7.615 278 678.94 7.581 65 678.31 7.574 66 677.56 7.566 75 676.88 7.558 83 676.31 7.552 85 675.81 7.546 90 675.31 7.541 86 673.63 7.522 160 672.31 7.507 71 671.94 7.503 69 671.63 7.500 66 671.06 7.493 64 670.56 7.488 66 670.06 7.482 62 669.69 7.478 65 669.38 7.475 60 619.94 6.923 169 331.13 3.698 1000 319.50 3.568 745
More Suppliers