-
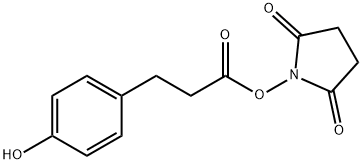
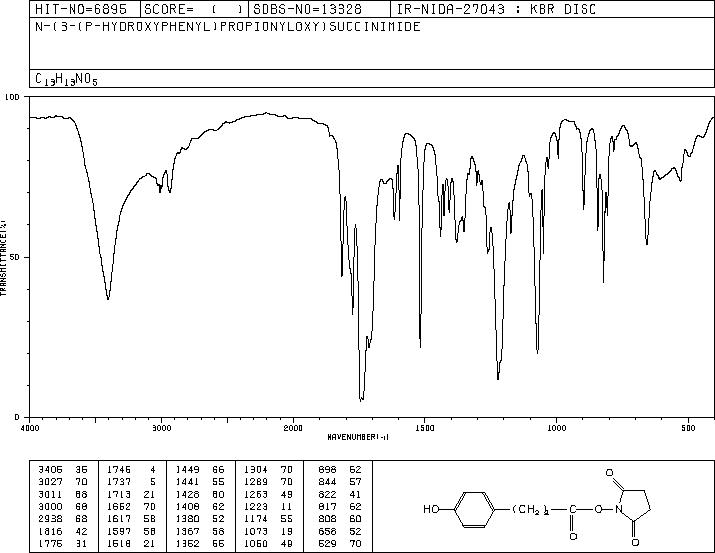
MS-NW-5474 N-(3-(p-hydroxyphenyl)propionyloxy)succinimide C13H13NO5 (Mass of molecular ion: 263)
Source Temperature: 180 °C Sample Temperature: 100 °C Direct, 75 eV
14.0 1.5 15.0 1.7 26.0 6.1 27.0 15.8 28.0 23.6 29.0 6.1 30.0 1.9 31.0 3.3 38.0 1.5 39.0 7.5 40.0 1.3 41.0 1.9 42.0 7.7 43.0 6.4 44.0 1.8 45.0 6.4 50.0 1.7 51.0 4.3 52.0 1.9 53.0 2.7 54.0 1.0 55.0 28.4 56.0 5.8 57.0 2.2 59.0 4.7 62.0 1.2 63.0 2.9 65.0 5.2 66.0 1.1 70.0 2.6 73.0 3.0 74.0 5.3 77.0 12.3 78.0 2.8 79.0 1.7 87.0 12.0 91.0 5.7 94.0 2.1 100.0 1.9 101.0 1.1 103.0 1.5 107.0 100.0 108.0 7.4 115.0 16.1 119.0 2.4 120.0 7.5 121.0 2.0 123.0 1.2 166.0 26.4 167.0 2.6
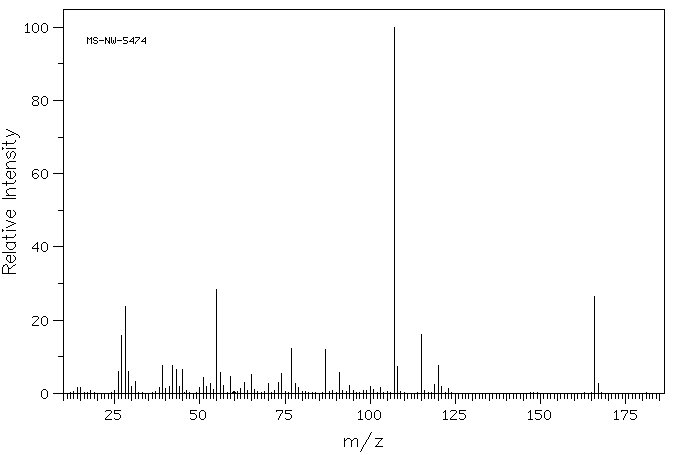
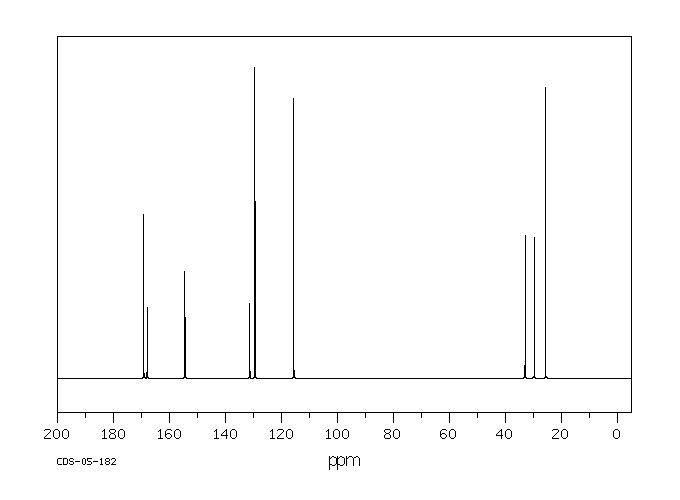
90 MHz in CDCl3
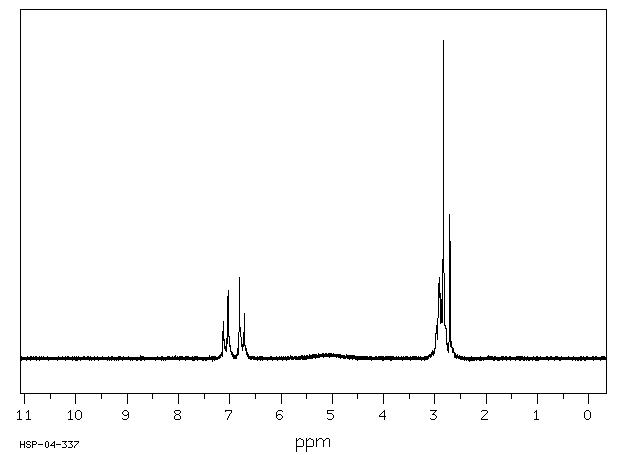
400 MHz in CDCl3
-
1H NMR 89.56 MHz C13 H13 N O5 0.015 g : 0.5 ml CDCl3 N-(3-(p-hydroxyphenyl)propionyloxy)succinimide 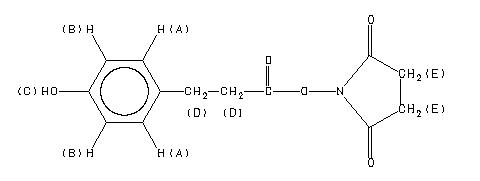
Assign. Shift(ppm)
A 7.06 B 6.77 C 5. D 3.00 to 2.86 E 2.828
Hz ppm Int.638.25 7.127 116 636.88 7.112 61 636.25 7.105 59 631.88 7.056 62 629.56 7.030 213 609.81 6.809 254 607.94 6.789 97 607.63 6.785 98 603.31 6.737 62 601.13 6.713 141 265.88 2.969 103 265.56 2.966 103 261.81 2.924 234 260.19 2.906 256 257.63 2.877 120 256.50 2.865 184 253.38 2.830 1000 250.44 2.797 105 249.75 2.789 99 249.19 2.783 102
-
1H NMR 399.65 MHz C13 H13 N O5 saturated in CDCl3 N-(3-(p-hydroxyphenyl)propionyloxy)succinimide 
Assign. Shift(ppm)
A 7.078 B 6.771 C 5.4 D 2.97 E 2.86 F 2.84
Hz ppm Int.2843.75 7.116 31 2843.14 7.115 33 2841.43 7.110 40 2840.45 7.108 44 2839.60 7.106 46 2838.75 7.104 53 2832.89 7.089 630 2824.46 7.068 714 2817.63 7.051 35 2817.38 7.050 35 2816.28 7.047 32 2719.60 6.805 30 2717.90 6.801 41 2716.80 6.798 48 2716.31 6.797 51 2715.94 6.796 53 2713.01 6.789 158 2710.21 6.782 841 2708.13 6.777 342 2705.69 6.771 120 2703.61 6.765 307 2701.66 6.761 752 2698.85 6.754 131 2695.56 6.745 40 1211.43 3.032 30 1210.57 3.030 32 1210.21 3.029 33 1209.84 3.028 33 1209.23 3.026 34 1208.62 3.025 35 1207.52 3.022 35 1206.79 3.020 36 1206.42 3.019 39 1206.05 3.018 38 1205.32 3.016 41 1204.71 3.015 40 1204.10 3.013 41 1203.49 3.012 47 1203.25 3.011 47 1201.90 3.008 55 1201.54 3.007 56 1200.93 3.005 62 1195.80 2.993 235 1188.84 2.975 491 1180.79 2.955 463 1177.00 2.946 70 1175.90 2.943 59 1175.29 2.941 55 1174.93 2.940 54 1174.32 2.939 55 1173.46 2.937 63 1173.10 2.936 64 1171.88 2.933 58 1171.39 2.932 60 1171.14 2.931 60 1170.78 2.930 61 1170.17 2.928 63 1169.68 2.927 60 1169.31 2.926 58 1168.58 2.925 56 1167.85 2.923 56 1167.48 2.922 55 1166.99 2.921 56 1166.50 2.919 58 1164.67 2.915 65 1163.94 2.913 72 1163.45 2.912 77 1162.96 2.910 81 1160.40 2.904 112 1159.55 2.902 107 1158.69 2.900 109 1158.08 2.898 108 1157.71 2.897 108 1150.88 2.880 590 1150.51 2.879 590 1146.48 2.869 306 1146.12 2.868 306 1144.29 2.864 537 1142.94 2.860 753 1134.52 2.839 1000 1123.05 2.811 163 1122.19 2.808 156 1121.83 2.808 153 1121.22 2.806 154 1116.82 2.795 60 1115.97 2.793 54 1114.75 2.790 48 1114.14 2.788 42 1113.77 2.787 42 1113.28 2.786 41 1112.43 2.784 37 1111.82 2.782 35 1111.45 2.782 35 1110.96 2.780 33 1110.60 2.779 30 1110.11 2.778 33
More Suppliers