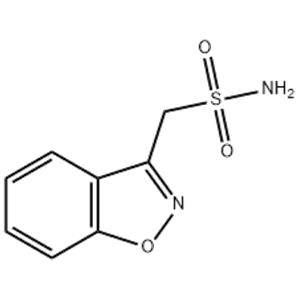
Zonisamide, the English name is Zonisamide, the chemical name is 1, 2-benzoisoxazol-3-methane sulfonamide, isa new broad-spectrum anti-epileptic drug developed by a large pharmaceutical company in Japan. Epilepsy is one of the common diseases of the nervous system in children. There are about 50 million epilepsy patients worldwide, and about 9 million epilepsy patients in China, most of whom start in childhood. About 20% to 30% of children with epilepsy cannot control their seizures after formal treatment with 2 or more antiepileptic drugs, which is called drug-resistant epilepsy or drug-resistant epilepsy. This product has brought more hope for the treatment of drug-resistant epilepsy and has received great attention. Studies have shown that this product is effective for many types of epilepsy carbuncle, including partial seizures of epilepsy carbuncle or secondary systemic seizures, systemic tonic-clonic seizures are effective. It is clinically used for the treatment of major seizures, minor seizures, localized seizures, epileptic status and psychomotor seizures with little side effects. It also has a preventive effect on mania, Parkinson's disease, and is used in the treatment of neuropathic pain and migraine. It was first approved for listing in Japan in 1989, in the United States in 2000 and in Europe in 2005.

 China
China