1/1
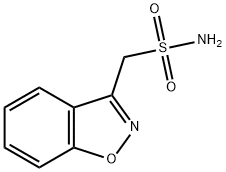
Zonisamide
$ 1.00
/1KG
- Min. Order1KG
- Purity99%
- Cas No68291-97-4
- Supply Ability10000KGS
- Update time2019-12-31

career henan chemical co
VIP7Y
 China
China
Since:2014-12-17
Address:702, Building 10, East District, National University Science and Technology Park, High tech Zone, Zh
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Zonisamide |
| CAS No | 68291-97-4 |
| EC-No | |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 10000KGS |
| Release date | 2019/12/31 |
hanne 721
▼
▲
Product Name:
Zonisamide
Synonyms:
(1,2-Benzisoxazol-3-yl)methanesulfonamide;Excemide;AD810, CI912;1,2-benzoxazol-3-ylmethanesulfonamide;indoxazen-3-ylmethanesulfonamide;Zonisamide, 1.0 mg/mL;BENZO[D]ISOXAZOL-3-YL-METHANESULFONAMIDE;1,2-BENZISOXAZOLE-3-METHANESULFONAMIDE
CAS:
68291-97-4
MF:
C8H8N2O3S
MW:
212.23
EINECS:
614-395-8
Product Categories:
Sulfur & Selenium Compounds;Calcium channel;Anticonvulsant;Aromatics;Ion channels;Zonegran;Heterocycles;Intermediates & Fine Chemicals;Neurochemicals;Pharmaceuticals
Mol File:
68291-97-4.mol
▼
▲
Zonisamide Chemical Properties
▼
▲
Melting point
275°C dec.
Boiling point
223°C (rough estimate)
density
1.4306 (rough estimate)
refractive index
1.5690 (estimate)
Fp
9℃
storage temp.
Store at +4°C
solubility
H2O: >5 mg/mL, soluble
pka
10.2(at 25℃)
form
solid
color
off-white
Merck
14,10192
InChIKey
UBQNRHZMVUUOMG-UHFFFAOYSA-N
CAS DataBase Reference
68291-97-4(CAS DataBase Reference)
▼
▲
Safety Information
▼
▲
Hazard Codes
Xn,T,F
Risk Statements
22-39/23/24/25-23/24/25-11
Safety Statements
7-16-36/37-45
RIDADR
UN1230 - class 3 - PG 2 - Methanol, solution
WGK Germany
3
RTECS
DE4930000
HS Code
2935904000
Hazardous Substances Data
68291-97-4(Hazardous Substances Data)
Toxicity
LD50 in mice, rats (mg/kg): 1892, 2001 orally; 1273, 2569 s.c.; 699, 733 i.p.; 604, 748 i.v. (Masuda, 1980)
▼
▲
MSDS Information
▼
▲
Provider
Language
1-(1,2-Benzoxazol-3-yl)methanesulphonamide
English
▼
▲
Zonisamide Usage And Synthesis
▼
▲
Description
Zonisamide is a new generation of sulfonamide anticonvulsant that is primarily used as supplemental therapy in treatment of partial seizures in combination with other antiepileptic medications. Besides, it is approved to be applied as an adjunctive therapy in adults suffering from infantile spasm, mixed seizure types of Lennox–Gastaut syndrome, myoclonic, and generalized tonic clonic seizure. Recent studies have proved that zonisamide can acts as a migraine preventative medication and is effective in several cases of neuropathic pain. In an open-label trial, zonisamide shows positive effects on attenuating the symptoms of tardive dyskinesia.
Zonisamide is a second-generation antiepileptic drug (AED) known with the proprietary brand name of Zonegran® (Eisai) in the UK and USA. It is assumed that zonisamide functions on the sodium and calcium channels in the brain cells, in which it controls electric-currents that are responsible for seizure activity. The FDA approved zonisamide in March 2000.
Zonisamide is a second-generation antiepileptic drug (AED) known with the proprietary brand name of Zonegran® (Eisai) in the UK and USA. It is assumed that zonisamide functions on the sodium and calcium channels in the brain cells, in which it controls electric-currents that are responsible for seizure activity. The FDA approved zonisamide in March 2000.
Indications
Epilepsy
Monotherapy of focal seizures with or without secondary generalization and adjunctive therapy of refractory focal seizures with or without secondary generalization.
Recommendations summarized from NICE (2012)
Monotherapy of focal seizures with or without secondary generalization and adjunctive therapy of refractory focal seizures with or without secondary generalization.
Recommendations summarized from NICE (2012)
- Seizure types: on referral to tertiary care (absence seizures, focal seizures, myoclonic seizures).
- Epilepsy types: on referral to tertiary care (absence syndromes, juvenile myoclonic epilepsy, idiopathic generalized epilepsy, benign epilepsy with centrotemporal spikes, panayiotopoulos syndrome, late- onset childhood occipital epilepsy).
Dose titration
Epilepsy
Monotherapy
100 mg od for 14 days, then increased by 100 mg every 14 days; usual maintenance 300 mg od (max. 500 mg daily).
Adjunctive therapy
25 mg bd for 7 days, 50 mg bd for 7 days, then increased by 100 mg every 7 days; usual maintenance 300– 500 mg daily, divided into 1 or 2 doses (dose to be increased every 14 days in patients who are not on carbamazepine, phenobarbital, phenytoin, or other potent inducers of cytochrome P450 enzyme CYP3A4).
Monotherapy
100 mg od for 14 days, then increased by 100 mg every 14 days; usual maintenance 300 mg od (max. 500 mg daily).
Adjunctive therapy
25 mg bd for 7 days, 50 mg bd for 7 days, then increased by 100 mg every 7 days; usual maintenance 300– 500 mg daily, divided into 1 or 2 doses (dose to be increased every 14 days in patients who are not on carbamazepine, phenobarbital, phenytoin, or other potent inducers of cytochrome P450 enzyme CYP3A4).
Plasma levels monitoring
Although plasma levels can be measured, and a therapeutic range has been postulated (10– 40 mg/ L), there is little evidence base for recommending routine measurement of plasma levels in clinical practice.
Cautions
- Patients with metabolic acidosis (consider dose reduction or discontinuation if metabolic acidosis develops).
- Patients with low body weight or poor appetite (monitor weight throughout treatment).
- Patients with risk factors or predisposition to nephrolithiasis.
- Elderly patients.
Interactions
With AEDs
There are no known specific interactions between alcohol and zonisamide and there are no specific foods that must be excluded from diet when taking zonisamide.
- Exposure to zonisamide is lower in epileptic patients receiving CYP3A4- inducing agents such as phenytoin, carbamazepine, and phenobarbital. These effects are unlikely to be of clinical significance when zonisamide is added to existing therapy; however, changes in zonisamide concentrations may occur if concomitant CYP3A4- inducing antiepileptic or other medicinal products are withdrawn, dose adjusted or introduced, an adjustment of the zonisamide dose may be required.
- Zonisamide should be used with caution in adult patients treated concomitantly with carbonic anhydrase inhibitors such as topiramate and acetazolamide, as there are insufficient data to rule out a possible pharmacodynamic interaction.
- Caution is advised when starting or stopping zonisamide treatment or changing the zonisamide dose in patients who are also receiving P- gp substrates such as digoxin and quinidine.
- If co- administration with rifampicin (a potent CYP3A4 inducer) is necessary, the patient should be closely monitored and the dose of zonisamide and other CYP3A4 substrates adjusted as needed.
There are no known specific interactions between alcohol and zonisamide and there are no specific foods that must be excluded from diet when taking zonisamide.
Special populations
Hepatic impairment
Renal impairment
Pregnancy
- Initially increase dose every 14 days in moderate impairment.
- Avoid in severe impairment.
Renal impairment
- Initially increase dose every 14 days in moderate impairment. • Discontinue if renal function deteriorates.
Pregnancy
- There are limited data from the use of zonegran in pregnant women and the potential risk in terms of reproductive toxicity for humans is unknown.
- Zonisamide must not be used during pregnancy unless it is required based on the clinical condition of the patient. In such cases, the dose of zonisamide should be monitored carefully during pregnancy and after birth, and adjustments made on a clinical basis.
- Zonisamide is excreted in human milk; the concentration in breastmilk is similar to maternal plasma. A decision must be made whether to discontinue breastfeeding or to discontinue/ abstain from zonisamide therapy.
Due to the long retention time of zonisamide in the body, breastfeeding must not be resumed until 1 month after zonisamide therapy is completed.
Behavioural and cognitive effects in patients with epilepsy
The behavioural profile of zonisamide in patients with epilepsy features specific problems, which can occur with high doses. The most commonly reported behavioural symptoms are depression, irritability, agitation, and psychosis. Cognitive deficits reported by patients treated with zonisamide mainly involve attention, concentration, and language domains (most effects occur at high doses).
Psychiatric use
Zonisamide does not have any approved indications in psychiatry. Initial findings from uncontrolled studies suggesting that zonisamide may be effective in the treatment of bipolar disorder did not find confirmation. There is preliminary evidence for possible usefulness of zonisamide in the treatment of obesity and psychotropicassociated weight gain, as well as alcohol dependence and withdrawal.
References
https://en.wikipedia.org/wiki/Zonisamide
http://www.medicinenet.com/zonisamide-oral/article.htm
https://pubchem.ncbi.nlm.nih.gov/compound/5734#section=Top
http://www.medicinenet.com/zonisamide-oral/article.htm
https://pubchem.ncbi.nlm.nih.gov/compound/5734#section=Top
Description
Zonisamide is a broad-spectrum antiepileptic effective in the treatment of refractory seizures. In cultured spinal cord neurons, zonisamide blocks the sustained firing of action potentials induced by depolarizing steps of current injected across the membrane.
Chemical Properties
Off-White Powder
Originator
Dainippon (Japan)
Uses
anticonvulsant;carbonic anhydrase inhibitor, repetitive firing of voltage-gated sodium channels and reduction of T-type calcium channel currents blocker
Uses
Sulfonamide antiseizure agent; blocks repetitive firing of voltagesensitive sodium channels and reduces voltage-sensitive T-type calcium currents. Heterocyclic methanesulfonide with anticonvulsant pro perties. The compound is under investigation for potential therapeutic use as an antiepileptic drug. Anticonvulsant.
Uses
muscarinic antagonist used as an antispasmodic
Uses
For use as adjunctive treatment of partial seizures in adults with epilepsy.
Definition
ChEBI: A 1,2-benzoxazole compound having a sulfamoylmethyl substituent at the 3-position.
Brand name
Zonegran (Dainippon Pharmaceutical Co., Japan);Exegran.
Biological Functions
Zonisamide has only recently been approved for use in the United States, although it has been available in Japan for several years. It is effective in partial complex and generalized tonic–clonic seizures and also appears to be beneficial in certain myoclonic seizures. It has a long half-life (about 60 hours) and requires about 2 weeks to achieve steady-state levels. It causes cerebellovestibular side effects similar to those of most other AEDs sharing its mechanism of action. In addition, it appears to cause an increased incidence of kidney stones.
General Description
Zonisamide, a sulfonamide-type anticonvulsant was recentlyapproved for adjunctive therapy in the treatment ofpartial seizures in adults with epilepsy.Zonisamide isprimarily metabolized by reductive ring cleavage of the 1,2-benzisoxazole ring to 2-sulfamoyl-acetyl-phenol. This biotransformation is mainly carried out by theintestinal bacteria rather than the mammalian cytosolicaldehyde oxidase suggested earlier.Again, because ofthe presence of a sulfonamide moiety in zonisamide molecule,precaution should be given to patients who have ahistory of hypersensitivity reactions toward sulfonamidedrugs and concomitant use of zonisamide with other carbonicanhydrase inhibitors should also be avoided.
Veterinary Drugs and Treatments
Zonisamide may be useful as an “add-on” drug for refractory epilepsy in dogs.
Company Profile Introduction
Established in 2014,Career Henan Chemical Co. is a manufacturerspecializing in the sale of fine chemicals. Mainly deals in the sales of: Pharmaceutical intermediates OLED intermediates: Pharmaceutical intermediates; OLED intermediates;