| CAS: | 102-70-5 |
| MF: | C9H15N |
| MW: | 137.22 |
| EINECS: | 203-048-2 |
| Product Categories: | Acyclic;Alkenes;Organic Building Blocks;Building Blocks;Chemical Synthesis;Organic Building Blocks |
| Mol File: | 102-70-5.mol |
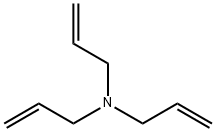 |
|
| Triallylamine Chemical Properties |
| Melting point | -70°C |
| Boiling point | 150-151 °C (lit.) |
| density | 0.79 g/mL at 25 °C (lit.) |
| vapor density | 4.73 (vs air) |
| vapor pressure | 90 mm Hg ( 80 °C) |
| refractive index | n20/D 1.451(lit.) |
| Fp | 87 °F |
| storage temp. | 2-8°C |
| pka | pK1:8.31(+1) (25°C) |
| form | clear liquid |
| color | Colorless to Yellow to Orange |
| Water Solubility | 250 g/100 mL |
| Stability: | Stable. Flammable. Incompatible with strong oxidizing agents. |
| InChIKey | VPYJNCGUESNPMV-UHFFFAOYSA-N |
| CAS DataBase Reference | 102-70-5(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Propen-1-amine, N,N-di-2-propenyl-(102-70-5) |
| EPA Substance Registry System | Triallylamine (102-70-5) |
| Triallylamine Usage And Synthesis |
| Chemical Properties | dark brown liquid |
| Chemical Properties | Triallylamine is a flammble liquid. Triallylamine can be detected at 0.5 ppm and is severely irritating at 75 ppm. |
| Uses | Triallylamine is used in organic synthesis.Triallylamine has been proposed as a catalyst for the production of polyesters and as an initiator for the polymerization of butadiene. |
| Uses | Triallylamine (TAA) reacts with primary aromatic amines in the presence of a ruthenium catalyst to form 2-ethyl-3-methylquinolines. TAA undergoes hydrozirconation followed by transmetalation with germanium tetrachloride to form 1-aza-5-germa-5-chlorobicyclo[3.3.3]undecane. This compound can react with Grignard or lithium reagents to form the corresponding 5-organo compounds. The cycloaddition of TAA to fluorinated 1,3,4-oxadiazoles affords octahydro-2,7-methanofuro[3,2-c]pyridines.
|
| Definition | ChEBI: Triallylamine is a tertiary amino compound. |
| Production Methods | Triallylamine is manufactured using allyl chloride and ammonia under heat and pressure. It is used as a solvent and in organic syntheses. |
| Application | Triallylamine (TAA) reacts with primary aromatic amines in the presence of a ruthenium catalyst to form 2-ethyl-3-methylquinolines.
TAA undergoes hydrozirconation followed by transmetalation with germanium tetrachloride to form 1-aza-5-germa-5-chlorobicyclo[3.3.3]undecane. This compound can react with Grignard or lithium reagents to form the corresponding 5-organo compounds.
The cycloaddition of TAA to fluorinated 1,3,4-oxadiazoles affords octahydro-2,7-methanofuro[3,2-c]pyridines. |
Product picture
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
