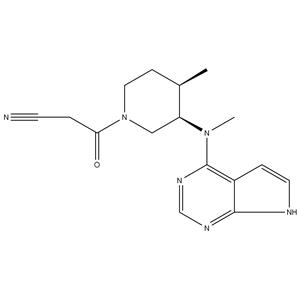
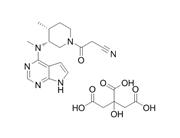
| Description | Tofacitinib is a JAK1/2/3 inhibitor with IC50s of 1, 20, and 112 nM, respectively. |
|---|
| Related Catalog | Signaling Pathways >> Epigenetics >> JAK Signaling Pathways >> JAK/STAT Signaling >> JAK Signaling Pathways >> Stem Cell/Wnt >> JAK Research Areas >> Inflammation/Immunology |
|---|
| Target | JAK3:1 nM (IC50) JAK2:20 nM (IC50) JAK1:112 nM (IC50) Rock-II:3400 nM (IC50) Lck:3870 nM (IC50) |
|---|
| In Vitro | Tofacitinib (CP-690550) citrate binds potentially at JAK3 and JAK2 as 2.2 nM and 5 nM (Kd). The report includes additional binding for Tofacitinib at Camk1 (Kd of 5,000 nM), DCamkL3 (Kd of 4.5 nM), Mst2 (Kd of 4,300 nM), Pkn1 (Kd of 200 nM), Rps6ka2 (Kin.Dom.2-C-terminal) (Kd of 1,400 nM), Rps6ka6 (Kin.Dom.2-C-terminal) (Kd of 1,200 nM), Snark (Kd of 420 nM), Tnk1 (Kd of 640 nM) and Tyk2 (Kd of 620 nM)[1]. |
|---|
| In Vivo | Animals that are treated with Tofacitinib show a significantly lower production of anti-drug antibodies (ADAs) compare with PEG-treated control mice (for five weeks after initial immunization, p<0.01, n=8). Moreover ADAs become detectable earliest on day 28. A difference of 1000- to 200-fold in titers to SS1P is apparent from days 21 through 35, respectively. Compare to SS1P, mice injected with keyhole limpet hemocyanin (KLH) generate a more rapid antibody response. Yet, the administration of Tofacitinib reduces anti-KLH titers compare to controls (p<0.05 on day 21, p<0.01 on day 28, respectively, n=5). Reductions in titers ranged from 5000- to 250-fold from days 21 through 28, respectively[2]. Based on previous dose-response studies, a daily dose of Tofacitinib of 6.2 mg/kg is selected to provide 80% inhibition of hind paw volume and plasma exposure capable of suppressing the JAK1 and JAK3 signaling pathways for >4 hours[3]. |
|---|
| Kinase Assay | Kinase activity is recorded via a competition binding assay of selected kinases that are fused to a proprietary tag. Measurements of the amount of kinase binds to an immobilized, active-site directed ligand in the presence and absence of the test compound (e.g., Tofacitinib) provide a % of DMSO control for binding of ligand. Activities between 0 and 10 are selected for Kd determinations. Dendrogram representations are generated by an in-house visualization tool designated PhyloChem[1]. |
|---|
| Cell Assay | Human CD4+ positive cells are enriched from peripheral blood mononuclear cells obtained from a healthy donor by magnetic separation (CD4+ MACS beads). CD4+ cells are activated for 3 days with plate bound anti-CD3 and anti CD28 antibodies (5 ug/mL each), and then expanded for another 4 days in the presence of IL-2 (50 U/mL). Cells are rested overnight in 1% RPMI, and pre-incubated with Tofacitinib or DMSO control for 1 hour at indicated concentrations (5 nM, 50 nM, 500 nM; DMSO concentration is equal in all preparations) and then activated with IL-2 (1000 u/mL) or IL-12 (100 ng/mL) for 15 minutes. Cells (10×106/condition) are lysed in 1% Triton-x lysis buffer and equal amounts of cell lysate are run in NuPage Bis-Tris gel (4-12% gradient). Proteins are transferred onto nitrocellulose membrane. Detection is done with indicated antibodies using Odyssey western blotting system[1]. |
|---|
| Animal Admin | Mice[2] Female BALB/c mice (6-8 weeks old) are used. Mice receive Tofacitinib in PEG300 (100 mg/mL) or vehicle alone (PEG300) by osmotic pump infusion (Alzet Model 2004, 0.25 μL/hour, 28 days). Four days prior to immunization, mice are anesthetized and their dorsal surface is shaved. A one cm incision is made on the back to create a subcutaneous pocket and insert the pump. The incision site is closed with wound clips. Mice are injected weekly (i.p.) with SS1P recombinant immunotoxin (RIT; 5 μg/mouse) beginning on day 0; control mice received injections of saline alone. Every week before SS1P or vehicle immunization, 50 μL of blood is drawn to obtain serum samples. Sera are stored at −80°C until analyzed. Rats[3] Adjuvant-induced arthritis (AIA) is induced in female Lewis rats. Rats are randomized according to hind paw volume and assigned to Tofacitinib or vehicle treatment regimens. Groups of 7-8 rats per treatment group, and normal naive rats (n=4 per group), are euthanized either 4 hours, 4 days, or 7 days after beginning treatment (days 16, 20, and 23 after immunization, respectively). Tofacitinib is suspended in 0.5% methylcellulose/0.025% Tween 20 for in vivo studies. Once-daily oral administration of vehicle or Tofacitinib (6.2 mg/kg) is initiated on day 16 following immunization and continued through day 23. Paw volumes are reassessed 4 and 7 days after the beginning of treatment (days 20 and 23 after immunization, respectively). For micro-computed tomography (micro-CT) imaging, as well as tartrate-resistant acid phosphatase (TRAP) staining in paw tissue, AIA is induced in a separate cohort of Lewis rats. |
|---|
| References | [1]. Jiang JK, et al. Examining the chirality, conformation and selective kinase inhibition of 3-((3R,4R)-4-methyl-3-(methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino)piperidin-1-yl)-3-oxopropanenitrile (CP-690,550). J Med Chem. 2008 Dec 25;51(24):8012-8. [2]. Onda M, et al. Tofacitinib suppresses antibody responses to protein therapeutics in murine hosts. J Immunol. 2014 Jul 1;193(1):48-55. [3]. LaBranche TP, et al. JAK inhibition with tofacitinib suppresses arthritic joint structural damage through decreased RANKL production. Arthritis Rheum. 2012 Nov;64(11):3531-42. [4]. Calama E, et al. Tofacitinib ameliorates inflammation in a rat model of airway neutrophilia induced by inhaled LPS. Pulm Pharmacol Ther. 2017 Apr;43:60-67. |
|---|

 China
China