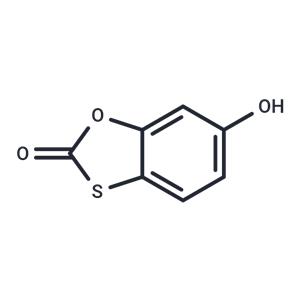
Tioxolone NEW
$ 30.00
/1mL
- Min. Order
- Purity97.79%
- Cas No4991-65-5
- Supply Ability10g
- Update time2026-02-02

TargetMol Chemicals Inc.
VIP6Y
 United States
United States
Enterprise Verified
Business Bank account
Basic Contact Infomation
Business Address
Trade Company



Chemical Properties
| Product Name | Tioxolone |
| CAS No | 4991-65-5 |
| EC-No | |
| Min. Order | |
| Purity | 97.79% |
| Supply Ability | 10g |
| Release date | 2026/02/02 |