| Description | Thiourea appears as white crystal/powder, is combustible, and on contact with fire, gives off irritating or toxic fumes/gases. Thiourea is a reducing agent used primarily in the production of bleached recycled pulp. In addition, it is also effective in the bleaching of stone groundwood, pressurised groundwood. Thiourea undergoes decomposition on heating and produces toxic fumes of nitrogen oxides and sulphur oxides. It reacts violently with acrolein, strong acids, and strong oxidants. The main application of thiourea is in textile processing and also is commonly employed as a source of sulphide. Thiourea is a precursor to sulphide to produce metal sulphides, for example, mercury sulphide, upon reaction with the metal salt in aqueous solution. The industrial uses of thiourea include production of flame-retardant resins and vulcanisation accelerators. Thiourea is used as an auxiliary agent in diazo paper, light-sensitive photocopy paper, and almost all other types of copy paper. Thiourea is used in many industrial applications, including as a chemical intermediate or catalyst, in metal processing and plating, and in photoprocessing. |
| Chemical Properties | Thiourea consists of colorless, lustrous crystals or powder with a bitter taste. |
| Chemical Properties | white crystals or powder |
| Uses | The product is wildly used in pharmaceutical industry, agricultural, chemicals, metallurgical industry, petroleum and so on. It is also main material for producing thiourea dioxide(CH1N2O2S). |
| Uses | Chaotropic agent; strong denaturant. Increases solubility and recovery of proteins |
| Uses | Used in determination of bismuth. |
| Uses | In animal glue liquifiers and silver tarnish removers. Photographic fixing agent and to remove stains from negatives; manufacture of resins; vulcanization accelerator; a reagent for bismuth, selenite ions. |
| Uses | Thiourea is used in the manufacture of resins,as a vulcanization accelerator, and as aphotographic fixing agent and to removestains from negatives. |
| Uses | The most common uses for thiourea have been for the production of thiourea dioxide (30%), in leaching of gold and silver ores (25%), in diazo papers (15%), and as a catalyst in the synthesis of fumaric acid (10%) (IARC 2001). It has also been used in the production and modification of synthetic resins. Other uses of thiourea are as a photographic toning agent, in hair preparations, as a drycleaning agent, in the synthesis of pharmaceuticals and pesticides, in boiler-water treatment, and as a reagent for bismuth and selenite ions. It has also been used in textile and dyeing auxiliaries, in the production of industrial cleaning agents (e.g., for photographic tanks and metal surfaces in general), for engraving metal surfaces, as an isomerization catalyst in the conversion of maleic to fumaric acid, in copper-refining electrolysis, in electroplating, and as an antioxidant. Other uses have included as a vulcanization accelerator, an additive for slurry explosives,as a viscosity stabilizer for polymer solutions, and as a mobility buffer in petroleum extraction. It is also used as an ingredient of consumer silver polishes (HPD 2009), and has been used in the removal of mercury from wastewater by chlorine-alkali electrolysis (IARC 1974, 2001, WHO 2003). |
| Production Methods | Thiourea is formed by heating ammonium thiocyanate at 170 °C (338 °F). After about an hour, 25% conversion is achieved. With HCl, thiourea forms thiourea hydrochloride; with mercuric oxide, thiourea forms a salt; and with silver chloride, it forms a complex salt. |
| Preparation | Thiourea is manufactured by heating ammonium thiocyanate at 140-145??C for about 4 hours; equilibrium is established when about 25% of the thiocyanate is converted to thiourea.Thiourea may also be prepared by the interaction of cyanamide and hydrogen sulphide:
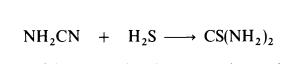
Thiourea closely resembles urea in that reaction with formaldehyde gives methylol derivatives and then resinous condensates which on continued heating yield network structures. Thiourea-formaldehyde resins are slower curing than urea-formaldehyde resins and the hardened products are more brittle and more water-resistant. At one time thiourea-formaldehyde resins were added to urea-formaldehyde resins to give mouldings and laminates with improved water-resistance. These mixed resins have now been largely superseded by melamine-formaldehyde resins which give products with better resistance to heat. |
| Definition | A colorless crystalline organic compound (the sulfur analog of urea). It is converted to the inorganic compound ammonium thiocyanate on heating. It is used as a sensitizer in photography and in medicine. |