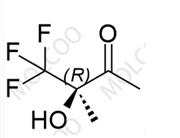
Suzetrigine Impurity
Product Code: S054002
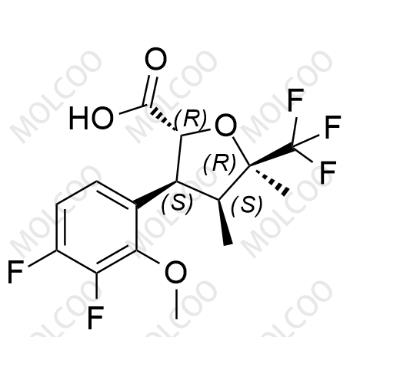
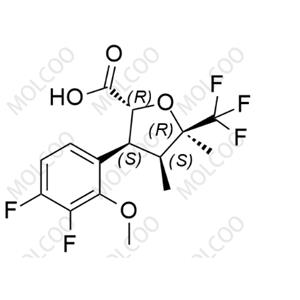
English Name: Suzetrigine Impurity 2
English Alias: (2R,3S,4S,5R)-3-(3,4-difluoro-2-methoxyphenyl)-4,5-dimethyl-5-(trifluoromethyl)tetrahydrofuran-2-carboxylic acid
CAS No.:2649470-87-9
Molecular Formula: C₁₅H₁₅F₅O₄
Molecular Weight: 354.27
High-Purity Reference Standard: As a reference standard for Suzetrigine Impurity 2, its structure is confirmed by NMR and HRMS with ≥99.0% purity (HPLC), suitable for qualitative and quantitative analysis of drug impurities.
Strong Stability: Stored at 2-8℃ in a sealed, light-protected environment, it has a shelf life of 24 months with batch-to-batch variation <0.5%, ensuring reliable detection data.
Quality Control: Used for HPLC and LC-MS detection of Impurity 2 in Suzetrigine API and formulations, controlling impurity content ≤0.1% according to ICH Q3A and other standards.
Process Optimization: Monitor the generation of Impurity 2 during Suzetrigine synthesis. Adjust esterification reaction temperature (e.g., controlled at 40-50℃) and catalyst dosage (e.g., triethylamine ratio) to reduce impurity generation to below 0.05%.
Stability Studies: Track changes in Impurity 2 content during accelerated stability tests (60℃/RH75%), evaluate its impact on drug stability, and provide data support for storage conditions and shelf life.
As a novel sodium channel inhibitor, Suzetrigine requires strict impurity control during R&D and production to ensure efficacy and safety. Impurity 2, a potential degradation or process impurity in its synthesis, contains a carboxylic acid group that may affect the drug's physicochemical properties and biological activity. With the improvement of international pharmaceutical regulatory standards (e.g., FDA, EMA), research on this impurity has become an important part of Suzetrigine's quality system.
Detection Technology: UPLC-MS/MS is used with a C18 column (1.7μm, 2.1×100mm) and 0.1% formic acid water-acetonitrile gradient elution, completing separation within 2.8 minutes with a detection limit of 0.008 ng/mL, 3 times more sensitive than traditional HPLC.
Formation Mechanism: Studies show that Impurity 2 may originate from the hydrolysis of the tetrahydrofuran ring during Suzetrigine synthesis or incomplete conversion of residual carboxylic acid intermediates in raw materials. Optimizing the purification process (e.g., using silica gel column chromatography) can reduce impurity content by over 60%.
Safety Evaluation: Preliminary cytotoxicity tests show an IC₅₀ of 25μM for Impurity 2 in HEK293 cells, lower than that of Suzetrigine (IC₅₀ 120μM), indicating low potential toxicity, but long-term exposure risks still need further evaluation through animal experiments.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China