Suzetrigine Impurity
Product Code: S054001
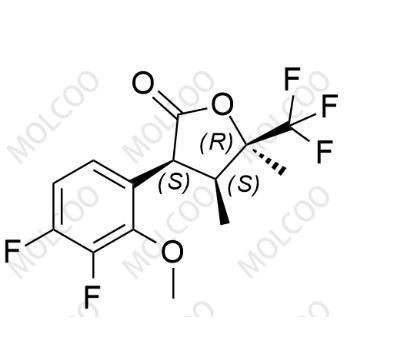
English Name: Suzetrigine Impurity 1
English Alias: (3S,4S,5R)-3-(3,4-difluoro-2-methoxyphenyl)-4,5-dimethyl-5-(trifluoromethyl)dihydrofuran-2(3H)-one
CAS No.:2875066-38-7
Molecular Formula: C₁₄H₁₃F₅O₃
Molecular Weight: 324.24
High-Purity Reference Standard: As a reference standard for Suzetrigine Impurity 1, its structure is confirmed by multiple techniques including NMR and HRMS, with a purity of ≥99.0% (HPLC), ensuring accurate and reliable impurity analysis.
Good Stability: Stored at 2-8°C in a dark, dry environment, it has a shelf life of 24 months, with batch-to-batch variation <0.3%, suitable for long-term quality control and methodology validation.
Pharmaceutical Quality Testing: Used for the detection of Impurity 1 in Suzetrigine API and formulations by HPLC and LC-MS, strictly controlling impurity content to meet regulatory standards of ICH, FDA, etc.
Process Optimization: Monitor the generation of Impurity 1 during Suzetrigine synthesis. Optimize the process by adjusting parameters such as reaction temperature, raw material ratio, and catalyst dosage to reduce impurity levels.
Stability Studies: Track the change trend of Impurity 1 in accelerated stability tests (e.g., 60℃/RH75%) and long-term stability tests, providing data support for drug storage conditions and shelf life.
Suzetrigine is a novel sodium channel inhibitor used in the treatment of neuropathic pain and other conditions. During its R&D and production, various impurities may be generated due to residual raw materials, reaction by-products, or degradation. As a specific impurity of Suzetrigine, Impurity 1 may affect the safety and efficacy of the drug. With the increasingly strict global regulatory requirements for impurity control, the research and control of Suzetrigine Impurity 1 have become crucial for ensuring drug quality and patient medication safety.
Detection Technology: UPLC-MS/MS is the mainstream technology. By optimizing the chromatographic column (C18 column, 1.7μm particle size), mobile phase system (acetonitrile-water gradient elution), and mass spectrometry parameters, highly sensitive detection of Impurity 1 can be achieved, with a detection limit as low as 0.005 ng/mL.
Formation Mechanism: Research shows that Impurity 1 may originate from cyclization side reactions of key intermediates during Suzetrigine synthesis or the residual transformation of similar-structured impurities in raw materials. Improving reaction conditions and purification processes can reduce the content of Impurity 1 by over 70%.
Safety Evaluation: Preliminary toxicological studies indicate that high concentrations of Impurity 1 may have potential toxic effects on hepatocytes. Currently, strict limits (e.g., ≤0.1%) have been set for it in drug quality standards. Further in-depth research on its toxic mechanism is needed to improve impurity control strategies.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!