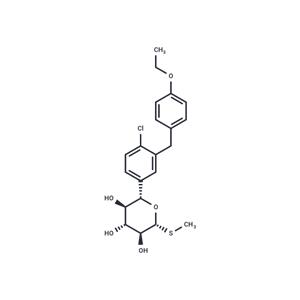
| Name | Sotagliflozin |
| Description | Sotagliflozin (LP-802034) is an orally bioavailable inhibitor of the sodium-glucose co-transporter subtype 1 (SGLT1) and 2 (SGLT2), with potential antihyperglycemic activity. |
| In vitro | LX4211 inhibits [14C]AMG uptake with IC50 of 62.0 nM for mouse SGLT1 and 0.6 nM for mouse SGLT2, respectively. [2] |
| In vivo | In mice, LX4211 (60 mg/kg, p.o.) reduces intestinal glucose absorption by inhibiting SGLT1, resulting in net increases in GLP-1 and PYY release and decreases in GIP release and blood glucose excursions. [2] In nonobese diabetes-prone mice with type 1 diabetes, Sotagliflozin (30 mg/kg) significantly improves glycemic control, without increasing the rate of hypoglycemia measurements. [3] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 50 mg/mL (117.66 mM)
H2O : < 1 mg/mL (insoluble or slightly soluble)
Ethanol : 14 mg/mL (32.9 mM)
|
| Keywords | inhibit | LX4211 | Sotagliflozin | Inhibitor | SGLT | LP 802034 | Sodium-dependent glucose cotransporters | LX 4211 | LP802034 |
| Inhibitors Related | Dapagliflozin ((2S)-1,2-propanediol, hydrate) | Ertugliflozin L-pyroglutamic acid | Dapagliflozin | Ipragliflozin | Tofogliflozin (hydrate) | Phlorizin | T-1095 | Phloretin | Sergliflozin A | Empagliflozin | Canagliflozin | Ertugliflozin |
| Related Compound Libraries | Membrane Protein-targeted Compound Library | EMA Approved Drug Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Bioactive Compounds Library Max | GPCR Compound Library | Anti-Cancer Drug Library |

 United States
United States