| Product Name | Sodium nitrite |
| CAS No | 7632-00-0 |
| EC-No | 231-555-9 |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | 50000KG/month |
| Release date | 2025/03/21 |
| CAS: | 7632-00-0 |
| MF: | NaNO2 |
| MW: | 69 |
| EINECS: | 231-555-9 |
| Product Categories: | Water Ttreatment Chemicals;Sodium nitrile;Inorganics;Q-S, Puriss p.a. ACS;Analytical Reagents for General Use;Puriss p.a. ACS;Inorganic Salts;Salts;Sodium Salts;SodiumMetal and Ceramic Science;Synthetic Reagents;ACS GradeSynthetic Reagents;Essential Chemicals;Routine Reagents;Other AdditivesVolumetric Solutions;Reference Material Potassium permanganateVolumetric Solutions;Allergens;By Reference Material;Cosmetics;S - Z;Salt Solutions;inorganic compound;Chemical Synthesis;Inorganic Salts;Materials Science;Metal and Ceramic Science;Sodium;Sodium Salts;Synthetic Reagents;Ultra-High Purity Materials;7632-00-0 |
| Mol File: | 7632-00-0.mol |
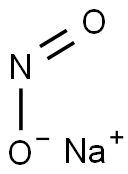 | |
| Sodium nitrite Chemical Properties |
| Melting point | 271 °C (lit.) |
| Boiling point | 320 °C |
| density | 2.17g/cm3 |
| storage temp. | 2-8°C |
| solubility | aqueous acid: 1 - 2μl acetic acid per ml H2Osoluble |
| form | powder |
| Specific Gravity | 2.168 |
| color | White or colorless |
| Odor | Odorless |
| PH | 9 (100g/l, H2O, 20℃) |
| PH Range | 9 |
| Water Solubility | 820 g/L (20 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,8648 |
| Stability: | Stable. Incompatible with reducing agents, strong oxidizing agents, organics and other flammable materials, finely powdered metals. Contact with combustible material may lead to fire. Hygroscopic. |
| InChIKey | LPXPTNMVRIOKMN-UHFFFAOYSA-M |
| CAS DataBase Reference | 7632-00-0(CAS DataBase Reference) |
| EPA Substance Registry System | Sodium nitrite (7632-00-0) |
| Sodium nitrite Usage And Synthesis |
| Physicochemical Properties | Chemical formula is NaNO2, in which N has a valency is + III.It is colorless or yellow crystal, the relative density is 2.168 (0℃), the melting point is 271℃, and it is decomposed when 320℃. It is soluble in water, and aqueous solution is alkaline because of nitrate hydrolysis. Sodium nitrite has the characteristics of reduction and oxidation and is mainly oxidation. In acidic solution, the main performance is oxidation. In alkaline solution or in case of strong oxidizing agent, its performance is reduction. With sulfur, phosphorus, organic matter and other friction or impact can cause combustion or explosion. Sodium nitrite can be placed in the air with the oxygen reaction, and gradually produce sodium nitrate: NaNO2+1/2O2=NaNO3. When using strong acidic sodium nitrite, it can be nitrited to nitric acid. Nitrite is very unstable, easily decomposed into nitrogen dioxide, nitric oxide and water. The nitrogen atoms and oxygen atoms all have a single pair of electrons, which can be used as ligands, and can be used as ligands to form complexes with many metal ions. Sodium nitrite is toxic, carcinogenic substances, using it must be attention. It is used in printing and dyeing industry and organic synthesis. Sodium nitrite is obtained by the reaction of sodium nitrate and lead in a total of hot condtion. NaNO3+Pb=NaNO2+PbO. The reaction mixture obtained by hot water treatment, filtration to remove insoluble lead oxide, concentration and crystallization of sodium nitrite crystal can be obtained.  Figure 1 white sodium nitrite crystal powder The information of this information is compiled by ChemicalBook Xiao Nan |
| Chemical Properties | Sodium nitrite is an inorganic compound with the chemical formula NaNO2. It is white or yellow patch on the orthorhombic crystal or powder. Micro salty and deliquescent. It is soluble in water and liquid ammonia, its aqueous solution is alkaline. It is also found at low concentrations in most vegetables. Spinach and lettuce can have some of the highest concentrations but all vegetables will contain some levels of sodium nitrite. It has been explored in human and veterinary medicines as a vasodilator, reducing blood pressure, and is also used as an antidote for cyanide poisoning. |
| Uses | Sodium nitrite is a commonly used meat preservative, particularly in cured meats such as ham, hot dogs, sausages, and bacon. The nitrite ion inhibits the growth of bacteria, particularly Clostridium botulinum, an organism that produces the deadly botulism toxin. Sodium nitrite is also used to treat packages of red meat, such as beef. Blood exposed to the air rapidly produces a brown color, but shoppers much prefer their meat purchases to look bright red.Thus,the meat is treated with sodium nitrite; the nitrite ion is reduced to nitrogen monoxide, which then reacts with the hemoglobin to form a very stable bright red compound.It is true that the nitrite will prevent bacterial growth in this circumstance as well, but these days, the meat is kept at temperatures low enough to inhibit bacteria.To persuade shoppers to prefer brownish rather than red meat will require a lot of re-education. Now that all meats are treated with sodium nitrite, there is concern that the cooking process will cause the nitrite ion to react with amines in the meat to produce nitrosamines,compounds containing the -NNO functional group. These compounds are known to be carcinogenic.However, as long as preserved meats are consumed in moderation, it is generally believed that the cancer risk is minimal. |
| toxicity | Due to the oxidizing property of sodium nitrite, ingestion of the substance can induce methemoglobinemia as quickly as one hour after ingestion. Methemoglobinemia occurs when the iron contained within hemoglobin is oxidized from its ferrous state (HgbFe2+) to its ferric state (HgbFe3+). When hemoglobin is in its ferric state, it is called methemoglobin and it is unable to act as a carrier to deliver oxygen to tissues. Symptoms of methemoglobinemia can include cyanosis and low SpO2 in the absence of respiratory distress, dizziness, syncope, dyspnea, fatigue, CNS depression, seizures, dysrhythmias, metabolic acidosis, cardiovascular collapse and death. Generally symptoms start to occur with methemoglobin levels > 15%. Sodium nitrite has a reported elimination half-life between 30-45 minutes when ingested or injected so does not tend to cause prolonged methemoglobinemia like that which is seen with dapsone. https://med.virginia.edu |
| Production | Sodium nitrite may be prepared by the thermal decomposition of sodium nitrate, but the reduction of nitrate is usually effected by stirring lead parings or copper filings into the molten salt: NaNO3+ Pb →PbO + NaNO2 After cooling, the mass is extracted with hot water,filtered and sodium nitrite crystallized after evaporation to small bulk. Industrially,sodium nitrite is formed by the action of nitrogen oxide (nitric oxide) and nitrogen dioxide together, obtained by the catalytic oxidation of ammonia, on sodium hydroxide or sodium carbonate solutions: NO+NO2+2OH- →2NO2-+ HO |
| Description | Sodium nitrite is similar in name and use to sodium nitrate. Both are preservatives used in processed meats, such as salami, hot dogs, and bacon. Sodium nitrite has been synthesized by several chemical reactions that involve the reduction of sodium nitrate. Industrial production of sodium nitrite is primarily by the absorption of nitrogen oxides into aqueous sodium carbonate or sodium hydroxide. Over the years, sodium nitrite has raised some concerns about its safety in foods, but it remains in use and there are indications that it may actually be healthy. Sodium nitrite was developed during the 1960s. In 1977, the US Department of Agriculture (USDA) considered banning it but the USDA’s final ruling on the additive came out in 1984, allowing its use. Studies in the 1990s indicated some adverse effects of sodium nitrite, for instance the potential to cause childhood leukemia and brain cancers. In the late 1990s, the National Toxicity Program (NTP) began a review of sodium nitrite and proposed listing sodium nitrite as a developmental and reproductive toxicant, but a report in 2000 by NTP proposed that sodium nitrite is not a toxic substance and removed it from the list of developmental and reproductive toxicants. It is now believed that it can help with organ transplants and leg vascular problems, while preventing heart attacks and sickle cell disease. |
| Chemical Properties | Sodium nitrite, NaN02, is a fire-hazardous, air-sensitive, yellowish white powder that is soluble in water and decomposes at temperatures above 320°C (608 °F). Sodium nitrite is used as an intermediate for dye stuffs and for pickling of meat, in dyeing of textiles, in rustproofing, in medicine, and as a reagent in organic chemistry. |
| Uses | Sodium nitrite is used to fix the colors in preserved fish and meats. It is also important(along with sodium chloride) in controlling the bacterium Clostridium botulinum, which causes botulism. Lunch meats, hams, sausages, hot dogs, and bacon are usually preserved this way. In medicines, it is a vasodilator, intestinal relaxant, bronchodilator, and an antidote to cyanide and hydrogen sulfide poisoning. Sodium nitrite is produced in the human body by the action of saliva on sodium nitrate, and is important in controlling bacteria in the stomach, to prevent gastroenteritis. The body produces more sodium nitrite than is consumed in food. Sodium nitrite can react with proteins in the stomach or during cooking, especially in high heat (such as frying bacon), to form carcinogenic N-nitrosamines.To prevent this, ascorbic acid or erythorbic acid is commonly added to cured meats. manufacture of diazo dyes, nitroso Compounds, and in many other processes of manufacture of organic chemicals; dyeing and printing textile fabrics; bleaching flax, silk, and linen. |
| Uses | Sodium Nitrite is the salt of nitrous acid that functions as an antimicrobial agent and preservative. it is a slightly yellow granular powder or nearly white, opaque mass or sticks. it is deliquescent in air. it has a solubility of 1 g in 1.5 ml of water. it is used in meat curing for color fixation and development of flavor. see nitrite. |
| Production Methods | Sodium nitrite, yellowish-white solid, soluble, formed (1) by reaction of nitric oxide plus nitrogen dioxide and sodium carbonate or hydroxide, and then evaporating, (2) by heating sodium nitrate and lead to a high temperature, and then extracting the soluble portion (lead monoxide insoluble) with H2O and evaporating. Used as an important reagent (diazotizing) in organic chemistry. |
| Definition | ChEBI: Sodium nitrite is an inorganic sodium salt having nitrite as the counterion. Used as a food preservative and antidote to cyanide poisoning. It has a role as an antimicrobial food preservative, an antihypertensive agent, a food antioxidant, a poison and an antidote to cyanide poisoning. It is a nitrite salt and an inorganic sodium salt. |
| General Description | A yellowish white crystalline solid. Noncombustible but will accelerate the burning of combustible material. If large quantities are involved in a fire or if the combustible material is finely divided, an explosion may result. If contaminated by ammonium compounds, spontaneous decomposition can occur and the resulting heat may ignite surrounding combustible material. Prolonged exposure heat may result in an explosion.Toxic oxides of nitrogen are produced in fires involving Sodium nitrite. Used as a food preservative, and to make other chemicals. |
| Air & Water Reactions | Soluble in water. |
| Reactivity Profile | Sodium nitrite is an oxidizing agent. Mixtures with phosphorus, tin(II) chloride or other reducing agents may react explosively [Bretherick 1979 p. 108-109]. If contaminated by ammonium compounds, spontaneous decomposition can occur and resulting heat may ignite surrounding combustible material. Reacts with acids to form toxic nitrogen dioxide gas. Mixing with liquid ammonia forms dipotassium nitrite, which is very reactive and easily explosive [Mellor 2, Supp. 3:1566 1963]. Melting together wilh an ammonium salt leads to a violent explosion [Von Schwartz 1918 p. 299]. A mixture with potassium cyanide may cause an explosion. Noncombustible but accelerates the burning of all combustible material. If large quantities are involved in fire or if the combustible material is finely divided, an explosion may result. When a little ammonium sulfate is added to fused potassium nitrite, a vigorous reaction occurs attended by flame [Mellor 2:702. 1946-47]. |
| Hazard | Dangerous fire and explosion risk when heated to 537C (1000F) or in contact with reducing materials; a strong oxidizing agent. Carcinogen in test animals; its use in curing fish and meat products is restricted to 100 ppm. |
Packing &shipping&Payment
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
Company Profile Introduction
-








