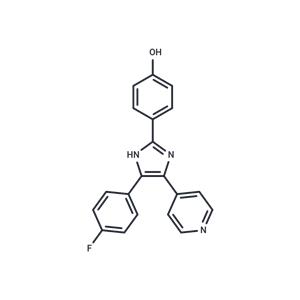
| Name | SB 202190 |
| Description | SB 202190 (FHPI) is a p38 MAPK inhibitor that inhibits p38α and p38β2 (IC50=50/100 nM) selectively and cell-permeably. SB 202190 has antitumor activity and also induces the differentiation of human embryonic stem cells into cardiomyocytes. |
| Cell Research | For transfection, A549 cells were seeded in 6-well plates to obtain 30% confluence at the time of transfection. Xtreme siRNA transfection reagent was used to transfect siRNA to a final concentration of 100 nM. Inhibition of gene expression by siRNA was determined after 48 hours by Western analysis. Cells were harvested, and the nuclear extract or total cell lysate was assayed for AP-1 DNA binding or Western blotting, respectively. HEK293T cells were cultured in complete DMEM. phCMV2-HA-MLK3 was transfected into HEK293T cells using genejammer transfection reagent using manufacturer's instructions. After 48 hours, cells were either untreated or treated with 5 or 10 μM SB202190 or SB203580 for 4 hours. Following treatment cell lysates were prepared using lysis buffer (50 mM Tris-HCl at pH 7.5, 5 mM EDTA, 150 mM NaCl, 1% Triton X-100, 50 mM NaF, 10 mM sodium pyrophosphate, 25 mM β- glycerophosphate, 1 mM PMSF, 30 μL/mL aprotinin, and 1 mM Na3VO4). 500 μg of total protein was immunoprecipitated with anti-HA-agarose conjugate. Phospho-MLK3 (Thr277/Ser281) was detected in western blotting using phosphospecific antibodies. The expression vector was transfected into HEK293T cells using Genejammer as stated earlier. After 48 hours, cell lysates was prepared and Flag-MKK7 was immunoprecipitated using anti-Flag-agarose conjugate. The Flag-MKK7 was used as a substrate for MLK3 kinase assay [3]. |
| Kinase Assay | All protein kinase activities were linear with respect to time in every incubation. Assays were performed either manually for 10 min at 30 °C in 50 μl incubations using [γ-32P]ATP or with a Biomek 2000 Laboratory Automation Workstation in a 96-well format for 40 min at ambient temperature in 25 μl incubations using [γ-32P]ATP. The concentrations of ATP and magnesium acetate were 0.1 mM and 10 mM respectively, unless stated otherwise. This concentration of ATP is 5–10-fold higher than the Km for ATP of most of the protein kinases studied in the present paper, but lower than the normal intracellular concentration, which is in the millimolar range. All assays were initiated with MgATP. Manual assays were terminated by spotting aliquots of each incubation on to phosphocellulose paper, followed by immersion in 50 mM phosphoric acid. Robotic assays were terminated by the addition of 5 μl of 0.5 M phosphoric acid before spotting aliquots on to P30 filter mats. All papers were then washed four times in 50 mM phosphoric acid to remove ATP, once in acetone (manual incubations) or methanol (robotic incubations), and then dried and counted for radioactivity [1]. |
| Animal Research | The pharmacological efficacy of SB-ULS-LZM was evaluated in the unilateral ischemia-reperfusion (I/R) rat model. At 2 h before the ischemia procedure, rats were injected with SB-ULS-LZM (32 mg/kg. conjugate, equivalent to 752 g/kg SB202190), vehicle (5% glucose), or free SB202190 (800 g/kg). SB-ULS-LZM was dissolved in 5% glucose, whereas SB202190 was dissolved in 20% hydroxypropyl-β-cyclodextrin solution with 5% dimethyl sulfoxide as described earlier. Compounds were administered i.v. via the penis vein as described above. Animals were allowed to recover and placed back into the cages until the induction of renal ischemia. Rats were operated, and the renal artery and vein were clamped under microscope to stop renal blood flow. After 45 min, clamps were removed, and reperfusion of the kidney was observed before closing of the wound. Sham-operated animals (n 3) received the same surgical procedure, with the exception of ischemia, and were included as a control group. After 4 days, animals were sacrificed, and blood samples were collected from the abdominal aorta. Kidneys were isolated after gently flushing the organs with saline and preserved in 4% formalin for preparation of paraffin-embedded sections or frozen in ice-cold isopentane for preparation of cryosections [2]. |
| In vitro | METHODS: Human Tenon fibroblasts were treated with SB 202190 (5-50 μM) and cell viability was measured by MTT assay.
RESULTS: SB 202190 is toxic to cells with an IC50 of 17.2 μM. [1]
METHODS: Human umbilical vein endothelial cells HUVEC were treated with SB 202190 (0.1-10 μM) for 6-48 h, and the expression levels of target proteins were detected by Western Blot method.
RESULTS: After incubation with SB 202190 for 24 h, conversion of LC3A/B-I to PE-coupled LC3A/B-II increased in a concentration-dependent manner. [2] |
| In vivo | METHODS: To investigate the role of p38 MAPK in mice with acute endotoxemia, SB 202190 (2 mg/kg) was injected intraperitoneally into C57BL/6 mice, followed by LPS (10 mg/kg) 30 min later.
RESULTS: Pretreatment with SB 202190 significantly reversed LPS-induced left ventricular depression and reduced LPS-induced mortality by decreasing TNF-α levels. [3]
METHODS: In order to detect anti-tumor activity in vivo, SB 202190 (5 mg/kg) and OSI-027 (10 mg/kg) were injected intraperitoneally into BALB/c mice bearing human CRC tumor SW620 once a day for ten days.
RESULTS: SB 202190 alone enhanced tumor proliferation and tumor load of SW620 xenografts. the combination of SB 202190 and OSI-027 significantly attenuated xenograft tumor growth. [4] |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 3.31 mg/mL (9.99 mM), Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately.
DMSO : 50 mg/mL (150.9 mM)
|
| Keywords | Apoptosis | colorectal | Autophagy | inhibit | SB 202190 | Inhibitor | memory | ATP | anti-cancer | learning | SB-202190 | pocket | spatial | deficits | p38 MAPK |
| Inhibitors Related | Stavudine | 5-Fluorouracil | Sodium 4-phenylbutyrate | L-Ascorbic acid | Hydroxychloroquine | Guanidine hydrochloride | Taurine | Tributyrin | Curcumin | Paeonol | Naringin | Gefitinib |
| Related Compound Libraries | Target-Focused Phenotypic Screening Library | Bioactive Compound Library | Pain-Related Compound Library | Kinase Inhibitor Library | Inhibitor Library | Stem Cell Differentiation Compound Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Compound Library | Anti-Cancer Active Compound Library |

 United States
United States