Welcome to inquire. Email: sales@anbuchem.com Whatsapp: +86 18638608485
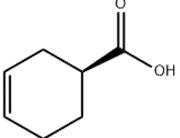
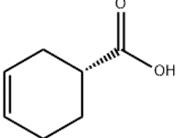
(S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID Basic information
Product Name: (S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID
Synonyms: (1S)-cyclohex-3-ene-1-carboxylic acid;(S)-Cyclohex-3-enecarboxylic acid;(S)-3-Cyclohexene-1-carboxylic Acid;(S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID;(S)-(?)-1,2,3,6-Tetrahydrobenzoic acid;(1S)-CYCLOHEX-3-ENE-1-CARBOXYLIC ACID(1S)-CYCLOHEX-3-ENE-1-CARBOXYLIC ACID;(1S)-3-Cyclohexene-1-carboxylic acid;(S)-(-)-3-Cyclohexene-1-carboxylic Acid
CAS: 5708-19-0
MF: C7H10O2
MW: 126.15
EINECS: 634-675-3
Product Categories: Edoxaban
Mol File: 5708-19-0.mol
(S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID Structure
(S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID Chemical Properties
Melting point 19°C(lit.)
Boiling point 118°C/6mmHg(lit.)
density 1.126±0.06 g/cm3(Predicted)
refractive index 1.4780 to 1.4820
storage temp. Keep in dark place,Inert atmosphere,Room temperature
form clear liquid
pka 4.67±0.20(Predicted)
color Colorless to Almost colorless
Safety Information
Hazard Codes C
Risk Statements 34
Safety Statements 26-36/37/39-45
RIDADR UN 3265 8 / PGIII
HazardClass 8
PackingGroup III
HS Code 2916200090
MSDS Information
(S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID Usage And Synthesis
Uses (S)-(-)-3-CYCLOHEXENECARBOXYLIC ACID can be used as organic synthesis intermediates and pharmaceutical intermediates, mainly used in laboratory research and development processes and chemical production processes.
Uses (S)-3-Cyclohexene-1-carboxylic Acid has been used as a reactant for the preparation of N-[(1R,2S,5S)-2-{[(5-chloroindol-2-yl)carbonyl]amino}-5-(dimethylcarbamoyl)cyclohexyl]-5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine-2-carboxamide hydrochloride, a potent and orally active direct inhibitor of factor Xa.
Synthesis 54.94 g of cyclohex-3-ene-1(S)-carboxylic acid (R)-(+)-methylbenzylamine salt and 18.2 volumes of H2O were charged to a flask at 20-25°C and stirred. To the mixture obtained further 6.7 volumes of H2O and 10 volumes of EtOAc were added. The mixture obtained was stirred for 15 min, the phases were separated and the organic layer was discarded. The aqueous layer was treated with 10 volumes of MTBE and 2.5 equivalents of 6M HCI were added slowly. The mixture obtained was stirred and two layers were formed, separated and the aqueous layer was extracted with MTBE. The organic layers obtained were combined and washed with aqueous, 30percent NaCl solution. The organic phase obtained was dried over Na2S04, filtered, and from the filtrate obtained solvent was removed in vacuo. Cyclohex-3-ene-1(S)-carboxylic acid in the form of a clear oil was obtained. Yield: 26.13 g (93.3percent of theory).

 China
China