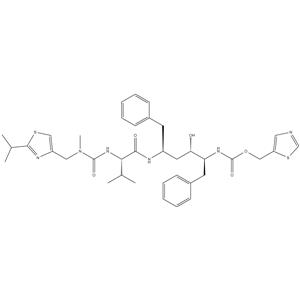
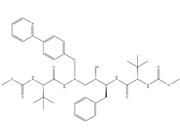
1/2
Ritonavir NEW
- Min. Order1KG
- Purity99%
- Cas No155213-67-5
- Supply Abilityg-kg-tons, free sample is available
- Update time2024-04-16

| Product Name | Ritonavir |
| CAS No | 155213-67-5 |
| EC-No | |
| Min. Order | 1KG |
| Purity | 99% |
| Supply Ability | g-kg-tons, free sample is available |
| Release date | 2024/04/16 |
1. Materials information
Names
| Name | ritonavir |
|---|---|
| Synonym | More Synonyms |
Ritonavir Biological Activity
| Description | Ritonavir is an inhibitor of HIV protease used to treat HIV infection and AIDS. |
|---|---|
| Related Catalog | Signaling Pathways >> Metabolic Enzyme/Protease >> HIV Protease Signaling Pathways >> Anti-infection >> HIV Research Areas >> Infection |
| In Vitro | Ritonavir is an inhibitor of CYP3A4 mediated testosterone 6β-hydroxylation with mean Ki of 19 nM and also inhibits tolbutamide hydroxylation with IC50 of 4.2 μM[1]. Ritonavir is found to be a potent inhibitor of CYP3A-mediated biotransformations (nifedipine oxidation with IC50 of 0.07 mM, 17alpha-ethynylestradiol 2-hydroxylation with IC50 of 2 mM; terfenadine hydroxylation with IC50 of 0.14 mM). Ritonavir is also an inhibitor of the reactions mediated by CYP2D6 (IC50=2.5 mM) and CYP2C9/10 (IC50=8.0 mM)[2]. Ritonavir results in an increase in cell viability in uninfected human PBMC cultures. Ritonavir markedly decreases the susceptibility of PBMCs to apoptosis correlated with lower levels of caspase-1 expression, decreases in annexin V staining, and reduces caspase-3 activity in uninfected human PBMC cultures. Ritonavir inhibits induction of tumor necrosis factor (TNF) production by PBMCs and monocytes in a time- and dose-dependent manner at nontoxic concentrations[3]. Ritonavir inhibits p-glycoprotein-mediated extrusion of saquinavir with an IC50 of 0.2 μM, indicating a high affinity of ritonavir for p-glycoprotein[4]. Ritonavir inhibits human liver microsomal metabolism of ABT-378 potently with Ki of 13 nM. Ritonavir combined with ABT-378 (at 3:1 and 29:1 ratios) inhibits CYP3A (IC50=1.1 and 4.6 μM), albeit less potently than Ritonavir (IC50=0.14 μM)[5]. |
| References | [1]. Eagling VA, et al. Differential inhibition of cytochrome P450 isoforms by the protease inhibitors, ritonavir, saquinavir and indinavir. Br J Clin Pharmacol. 1997 Aug;44(2):190-4. [2]. Kumar GN, et al. Cytochrome P450-mediated metabolism of the HIV-1 protease inhibitor ritonavir (ABT-538) in human liver microsomes. J Pharmacol Exp Ther. 1996 Apr;277(1):423-31. [3]. Weichold FF, et al. HIV-1 protease inhibitor ritonavir modulates susceptibility to apoptosis of uninfected T cells. J Hum Virol. 1999 Sep-Oct;2(5):261-9. [4]. Drewe J, et al. HIV protease inhibitor ritonavir: a more potent inhibitor of P-glycoprotein than the cyclosporine analog SDZ PSC 833. Biochem Pharmacol. 1999 May 15;57(10):1147-52. [5]. Kumar GN, et al. Potent inhibition of the cytochrome P-450 3A-mediated human liver microsomal metabolism of a novel HIV protease inhibitor by ritonavir: A positive drug-drug interaction. Drug Metab Dispos. 1999 Aug;27(8):902-8. |
Chemical & Physical Properties
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 947.0±65.0 °C at 760 mmHg |
| Melting Point | 120-122°C |
| Molecular Formula | C37H48N6O5S2 |
| Molecular Weight | 720.944 |
| Flash Point | 526.6±34.3 °C |
| Exact Mass | 720.312744 |
| PSA | 202.26000 |
| LogP | 5.28 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.600 |
| Storage condition | -20°C Freezer |
MSDS
Ritonavir MSDS(Chinese) |
Safety Information
| Symbol |  GHS07 |
|---|---|
| Signal Word | Warning |
| Hazard Statements | H302 + H312 + H332 |
| Precautionary Statements | P261-P280-P301 + P312 + P330 |
| Hazard Codes | Xi |
| Risk Phrases | R36/38:Irritating to eyes and skin . |
| Safety Phrases | 26-37/39 |
| RIDADR | NONH for all modes of transport |
| RTECS | XA5310000 |
| HS Code | 2942000000 |
Synthetic Route
Previous 1/3 Next![2,5-DIOXOPYRROLIDIN-1-YL N-{N-[(2-ISOPROPYL-1,3-THIAZOL-4-YL)METHYL]-N-METHYLCARBAMOYL}-L-VALINATE structure](https://www.chemsrc.com/caspic/082/224631-15-6.png) 2,5-DIOXOPYRROL... CAS#:224631-15-6  (2S,3S,5S)-5-Am... CAS#:144164-11-4 ~% 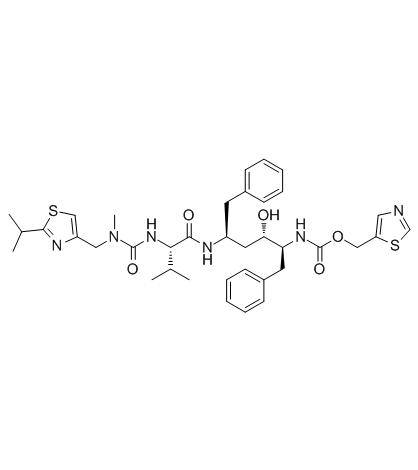 Ritonavir CAS#:155213-67-5 |
| Literature: WO2006/90264 A1, ; Page/Page column 23-24 ; |
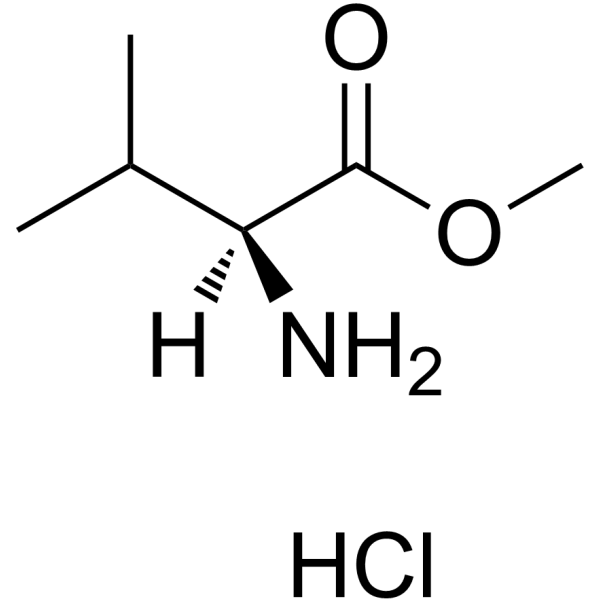 H-Val-OmeHCl CAS#:6306-52-1 ~%  Ritonavir CAS#:155213-67-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 4 p. 602 - 617 |
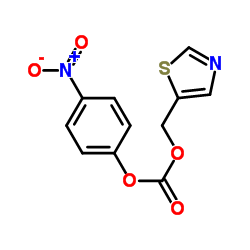 4-Nitrophenyl 1... CAS#:144163-97-3 ~%  Ritonavir CAS#:155213-67-5 |
| Literature: Journal of Medicinal Chemistry, , vol. 41, # 4 p. 602 - 617 |
Precursor & DownStream
| Precursor 8 | Previous 1/2 Next |
|---|---|
| |
| DownStream 0 | |
Customs
| HS Code | 2942000000 |
|---|
Articles39
More Articles| In vitro and in vivo release characteristics of Tacrolimus (FK506) from an episcleral drug-delivery implant. J. Ocul. Pharmacol. Ther. 30(8) , 670-80, (2014) To investigate the in vitro and in vivo release characteristics of Tacrolimus (FK506) from an episcleral drug-delivery implant.For in vitro experiments, Tacrolimus-loaded implants (0.5 mL; at concentr... | |
| Cell-free microfluidic determination of P-glycoprotein interactions with substrates and inhibitors. Pharm. Res. 31(12) , 3415-25, (2014) The membrane protein P-glycoprotein (P-gp) plays key roles in the oral bioavailability of drugs, their blood brain barrier passage as well as in multidrug resistance. For new drug candidates it is man... | |
| Molecular cloning and functional characterization of a rainbow trout liver Oatp. Toxicol. Appl. Pharmacol. 280(3) , 534-42, (2014) Cyanobacterial blooms have an impact on the aquatic ecosystem due to the production of toxins (e.g. microcystins, MCs), which constrain fish health or even cause fish death. However the toxicokinetics... |
Synonyms
| [5S-(5R*,8R*,10R*,11R*)]-10-Hydroxy-2-methyl-5-(1-methylethyl)-1-[2-(1-methylethyl)-4-thiazolyl]-3,6-dioxo-8,11-bis(phenylmethyl)-2,4,7,12-tetraazatridecan-13-oic acid 5-thiazolylmethyl ester |
| Norvir |
| Ritonavir |
| EINECS 208-127-9 |
| N-[(2S,4S,5S)-4-Hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl]-N-{[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)carbamoyl}-L-valinamide |
| Ritonavi |
| Carbamic acid, N-[(1S,2S,4S)-2-hydroxy-4-[[(2S)-3-methyl-2-[[[methyl[[2-(1-methylethyl)-4-thiazolyl]methyl]amino]carbonyl]amino]-1-oxobutyl]amino]-5-phenyl-1-(phenylmethyl)pentyl]-, 5-thiazolylmethyl ester |
| ABT-538 |
| (1E,2S)-N-[(2S,4S,5S)-4-Hydroxy-5-{(E)-[hydroxy(1,3-thiazol-5-ylmethoxy)methylene]amino}-1,6-diphenyl-2-hexanyl]-2-[(E)-(hydroxy{[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)amino}methylene)amino]-3-methylbutanimidic acid |
| 1,3-Thiazol-5-ylmethyl-[(1S,2S,4S)-1-benzyl-2-hydroxy-4-({(2S)-3-methyl-2-[(methyl{[2-(1-methylethyl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]butanoyl}amino)-5-phenylpentyl]carbamat |
| N-[(1S,3S,4S)-1-benzyl-3-hydroxy-5-phenyl-4-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}pentyl]-N-(methyl{[2-(1-methylethyl)-1,3-thiazol-4-yl]methyl}carbamoyl)-L-valinamide |
| [(1S,2S,4S)-1-benzyl-2-hydroxy-4-({(2S)-3-méthyl-2-[(méthyl{[2-(1-méthyléthyl)-1,3-thiazol-4-yl]méthyl}carbamoyl)amino]butanoyl}amino)-5-phénylpentyl]carbamate de 1,3-thiazol-5-ylméthyle |
| Butanimidic acid, N-[(1S,3S,4S)-3-hydroxy-4-[[(1E)-hydroxy(5-thiazolylmethoxy)methylene]amino]-5-phenyl-1-(phenylmethyl)pentyl]-2-[[(1E)-hydroxy[methyl[[2-(1-methylethyl)-4-thiazolyl]methyl]amino]methylene]amino]-3-methyl-, (1E,2S)- |
| N-[(2S,4S,5S)-4-Hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}-2-hexanyl]-N-{[(2-isopropyl-1,3-thiazol-4-yl)methyl](methyl)carbamoyl}-L-valinamide |
| carbamic acid, [(1S,2S,4S)-2-hydroxy-4-[[(2S)-3-methyl-2-[[[methyl[[2-(1-methylethyl)-4-thiazolyl]methyl]amino]carbonyl]amino]-1-oxobutyl]amino]-5-phenyl-1-(phenylmethyl)pentyl]-, 5-thiazolylmethyl es |
| N-[(2S,4S,5S)-4-hydroxy-1,6-diphenyl-5-{[(1,3-thiazol-5-ylmethoxy)carbonyl]amino}hexan-2-yl]-N2-(methyl{[2-(propan-2-yl)-1,3-thiazol-4-yl]methyl}carbamoyl)-L-valinamide |
| Liponavir Core |
| 1,3-thiazol-5-ylmethyl [(1S,2S,4S)-1-benzyl-2-hydroxy-4-({(2S)-3-methyl-2-[(methyl{[2-(1-methylethyl)-1,3-thiazol-4-yl]methyl}carbamoyl)amino]butanoyl}amino)-5-phenylpentyl]carbamate |
| A-84538 ABT-538 Abbott 84538 |
| Norvi |
| (2S,3S,5S)-5-[N-[N-[[N-methyl-N-[(2-isopropyl-4-thiazolyl)methyl]amino]carbonyl]valinyl]amino]-2-[N-[(5-thiazolyl)methoxycarbonyl]amino]-1,6-diphenyl-3-hydroxyhexane |
| RITONA |
| MFCD04115732 |
| carbamic acid, [(1S,2S,4S)-2-hydroxy-4-[[(2S)-3-methyl-2-[[[methyl[[2-(1-methylethyl)-4-thiazolyl]methyl]amino]carbonyl]amino]-1-oxobutyl]amino]-5-phenyl-1-(phenylmethyl)pentyl]-, 5-thiazolylmethyl ester |
2. Packaging of materials
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping & Delivery
By Express
Provide door to door service
Suitable for goods under 50kg
Delivery: 3-7 days
Cost: low cost

By Air
Provide airport to airport service
Suitable for goods over 50kg
Delivery: 3-14 days
Cost: high cost

By Sea
Provide seaport to seaport service
Suitable for goods over 100kg
Delivery: 2-45 days
Cost: low cost

4. Contact information
For more details, pls contact us freely.
Email address: Tina@fdachem.com
Mob: 86 13213167925
WhatsApp/Skype/Wechat/LINE: 86 13213167925
Company Profile Introduction
Henan Fengda Chemical Co., Ltd. is located in the High-tech Development Zone of Henan Province. Specializing in the production and sales of various fine chemical products required for industrial production, including chemical raw materials, organic raw materials, petrochemicals, chemical reagents, solvents, catalysts, and additives, etc.