| CAS: | 737789-87-6 |
| MF: | C29H27F2N7O5S |
| MW: | 623.63 |
| EINECS: |
|
| Product Categories: | API;737789-87-6 |
| Mol File: | 737789-87-6.mol |
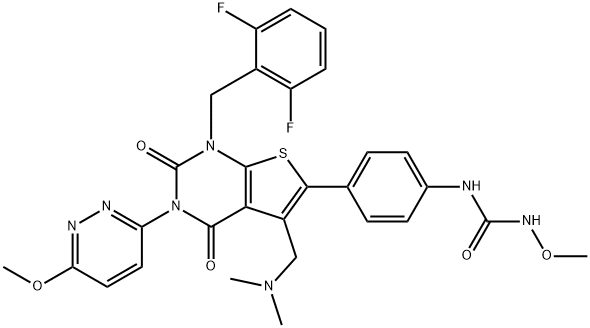 |
|
| Relugolix Chemical Properties |
| Melting point | 228 °C (decomp)(Solv: ethyl acetate (141-78-6); tetrahydrofuran (109-99-9)) |
| density | 1.442±0.06 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | DMSO:20.0(Max Conc. mg/mL);32.1(Max Conc. mM)
Ethanol:1.0(Max Conc. mg/mL);1.6(Max Conc. mM) |
| form | A crystalline solid |
| pka | 13.17±0.70(Predicted) |
| InChIKey | AOMXMOCNKJTRQP-UHFFFAOYSA-N |
| SMILES | N(C1=CC=C(C2SC3=C(C=2CN(C)C)C(=O)N(C2=NN=C(OC)C=C2)C(=O)N3CC2=C(F)C=CC=C2F)C=C1)C(NOC)=O |
| Relugolix Usage And Synthesis |
| Description | Relugolix (TAK-385) is a potent, orally active, nonpeptidic gonadotropin-releasing hormone (GnRH) antagonist. It possesses high affinity and potent antagonistic activity for human receptor (binding IC50=0.33 nM) and monkey receptor (IC50=0.32 nM) compared with TAK-013 (HY-100209). Relugolix is used for the study of sex-hormone-dependent diseases, such as including endometriosis, uterine fibroids and prostate cancer et al. |
| Uses | Relugolix is a highly selective, oral, nonpeptide GnRH antagonist being investigated as a possible prostate cancer treatment |
| Mechanism of action | The mechanism of action of relugolix is as a Gonadotropin Releasing Hormone Receptor Antagonist, and Cytochrome P450 3A Inducer, and Cytochrome P450 2B6 Inducer, and Breast Cancer Resistance Protein Inhibitor, and P-Glycoprotein Inhibitor. The physiologic effect of relugolix is by means of Decreased GnRH Secretion. |
| Pharmacokinetics | Relugolix is a selective antagonist of the gonadotropin-releasing hormone receptor (GnRHR) (IC50 = 0.12 nM).A single oral administration of relugolix at a dose of 3 mg/kg has been found to suppress luteinizing hormone (LH) levels for more than 24 hours in castrated cynomolgus monkeys, indicating a long duration of action. The drug (80–160 mg/day) has been found to reduce testosterone levels to sustained castrate levels in men with once-daily administration.[8] Lower dosages (10–40 mg/day) are being studied in the treatment of endometriosis and uterine fibroids to achieve partial sex hormone suppression. The reasoning behind partial suppression for these conditions is to reduce the incidence and severity of menopausal symptoms such as hot flushes and to avoid bone mineral density changes caused by estrogen deficiency that can eventually lead to osteoporosis. |
| Clinical Use | Relugolix was first approved in Japan in 2019, under the brand name Relumina, for the symptomatic treatment of uterine fibroids, and more recently by the United States' FDA in 2020, under the brand name Orgovyx, for the treatment of advanced prostate cancer. Relugolix has also been studied in the symptomatic treatment of endometriosis. |
Product picture
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock

 Japan
Japan








 Company information
Company information Advantage
Advantage


