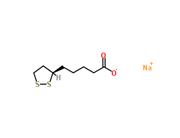
(R)-(+)-Lipoic acid is an endogenous antioxidant that can eliminate free radicals in the body. It promotes the utilization of glucose to synthesize vitamin C and aids in the synthesis of glutathione. It effectively removes melanin and assists coenzymes in physiological metabolism that enhances the body's immune system. (R)-(+)-Lipoic acid also possesses anti-inflammatory properties by inhibiting the activity of kinases, transforming factors, tumor necrosis factors, and collagenases, which contribute to its anti-aging effects. (R)-(+)-Lipoic acid preserves and regenerates other antioxidants, making it beneficial for maintaining good health. Currently, it is widely used in the prevention and treatment of heart disease, diabetes, liver disease, and Alzheimer's disease. The two enantiomers of (R)-(+)-Lipoic acid exhibit different biological activities. The R-enantiomer is much more effective than the S-enantiomer, possibly due to the fact that a large amount of R-enantiomer can enter cells and mitochondria through cell membranes and mitochondrial membranes to be reduced to dihydro(R)-(+)-Lipoic acid during the metabolism of (R)-(+)-Lipoic acid, whereas only a small amount of S-enantiomer enters cells and undergoes reduction. Dihydro(R)-(+)-Lipoic acid possesses stronger antioxidant capacity than (R)-(+)-Lipoic acid, and the regeneration of endogenous antioxidants and repair of oxidative damage both require the form of dihydro(R)-(+)-Lipoic acid.
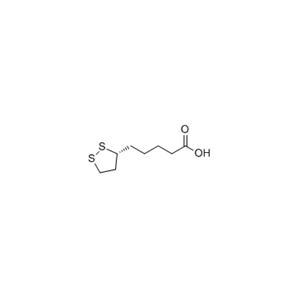

 China
China