| Name | Nintedanib |
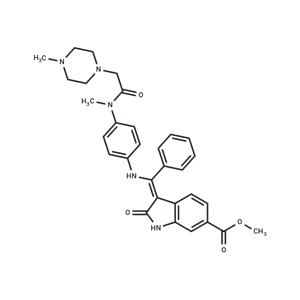
| Description | Nintedanib (Intedanib) is a triple vascular kinase inhibitor that inhibits VEGFR1, VEGFR2, and VEGFR3 (IC50=34/13/13 nM), FGFR1, FGFR2, and FGFR3 (IC50=69/37/108 nM), PDGFRα, and PDGFRβ (IC50=59/65 nM). Nintedanib has antitumor activity and inhibits tumor growth by inhibiting angiogenesis. |
| Cell Research | HUVEC, HUASMC, and BRP were cultured as described above. Two hours before the addition of ligands, BIBF 1120 was added to the cultures. Cell lysates were generated according to standard protocols. Western blotting was done using standard SDS-PAGE methods, loading 50 to 75 μg of protein per lane, with detection by enhanced chemiluminescence. Total and phosphorylated mitogen-activated protein kinase (MAPK) was analyzed using monoclonal antibodies M3807 and M8159. Total Akt was detected using the polyclonal antibody and phosphorylated Akt (Ser473) was analyzed with the monoclonal antibody. Cleaved caspase-3 was detected with the monoclonal antibody [1]. |
| Kinase Assay | The cytoplasmic tyrosine kinase domain of VEGFR-2 (residues 797–1355 according to sequence deposited in databank SWISS-PROT P35968) was cloned into pFastBac fused to GST and extracted as described in supplementary methods. Enzyme activity was assayed using standard conditions using a random polymer (Glu/Tyr 4:1) and in the presence of 100 μmol/L ATP (for details, see supplementary methods). For all other kinase assays, the entire cytoplasmic domains of the receptors (from the end of the transmembrane to the COOH terminus) were cloned into pFastBac vector containing GST and assayed under standard conditions [1]. |
| Animal Research | Five-week-old to 6-wk-old athymic NMRI-nu/nu female mice (21–31 g) were purchased from Harlan. After acclimatization, mice were inoculated with 1 to 5 × 10^6 (in 100 μL) FaDu, Caki-1, SKOV-3, H460, HT-29, or PAC-120 cells s.c. into the right flank of the animal. F344 Fischer rats after acclimatization were injected with 5 × 10^6 (in 100 μL) GS-9L cells s.c. into the right flank of the animal. For pharmacokinetic analysis, blood was isolated at indicated time points from the retroorbital plexus of mice and plasma was analyzed using high-performance liquid chromatography-mass spectrometry methodology [1]. |
| In vitro | METHODS: Human nasopharyngeal carcinoma cells CNE-2, HNE-1 and HONE-1 were treated with Nintedanib (0.078-10 µM) for 72 h, and cell viability was measured by CCK8 assay.
RESULTS: Nintedanib significantly inhibited the growth of CNE-2, HNE-1 and HONE-1 cell lines in a dose-dependent manner with IC50 values of 4.16 µM, 5.62 µM and 6.32 µM, respectively. [1]
METHODS: Human endothelial cells HUVEC, smooth muscle cells HUASMC and bovine pericytes were treated with Nintedanib (0.03-1 µM) for 2 h, and the expression levels of target proteins were detected by Western Blot.
RESULTS: Nintedanib inhibited ligand-dependent phosphorylation of MAPK and Akt in HUVEC, HUASMC and bovine pericytes. [2] |
| In vivo | METHODS: To test the antitumor activity in vivo, Nintedanib (10-100 mg/kg) was administered by gavage to athymic NMRI-nu/nu mice bearing the human head and neck small cell carcinoma tumor FaDu or the human renal carcinoma tumor Caki-1 once daily for 23-35 days.
RESULTS: Nintedanib inhibited tumor growth in FaDu and Caki-1. [2]
METHODS: To test the antitumor activity in vivo, Nintedanib (40 mg/kg) and TFTD (150 mg/kg) were administered intraperitoneally to BALB/c nu/nu mice bearing tumors of DLD-1, DLD-1/5-FU, HT-29, or HCT116 twice daily for two weeks.
RESULTS: At day 15, Nintedanib and TFTD monotherapy resulted in a significant reduction in tumor growth in vivo. The combination therapy exhibited greater anti-tumor activity than the two monotherapies. [3] |
| Storage | keep away from direct sunlight | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | 10% DMSO+40% PEG300+5% Tween 80+45% Saline : 0.6 mg/mL (1.11 mM), Please add co-solvents sequentially, clarifying the solution as much as possible before adding the next one. Dissolve by heating and/or sonication if necessary. Working solution is recommended to be prepared and used immediately.
Ethanol : 3 mg/mL (5.55 mM)
DMSO : 10 mg/mL (18.53 mM), Sonication is recommended.
H2O : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | FGFR | PDGFR | Inhibitor | Platelet-derived growth factor receptor | Fibroblast growth factor receptor | Vascular endothelial growth factor receptor | VEGFR | Nintedanib | BIBF1120 | inhibit | BIBF-1120 |
| Inhibitors Related | Ribociclib | Gilteritinib | Amlexanox | Regorafenib monohydrate | Sorafenib | Ferulic Acid | Regorafenib | Sorafenib tosylate | Formononetin | Imatinib | Pazopanib | Axitinib |
| Related Compound Libraries | Bioactive Compound Library | Membrane Protein-targeted Compound Library | EMA Approved Drug Library | Tyrosine Kinase Inhibitor Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Kinase Inhibitor Library | Bioactive Compounds Library Max | Anti-Cancer Active Compound Library |

 United States
United States