Product Number: N030001
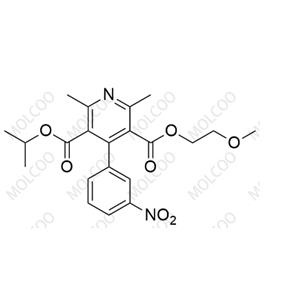
English Name: Nimodipine EP Impurity A
English Alias: 3-isopropyl 5-(2-methoxyethyl) 2,6-dimethyl-4-(3-nitrophenyl)pyridine-3,5-dicarboxylate
CAS Number: 85677-93-6
Molecular Formula: C₂₁H₂₄N₂O₇
Molecular Weight: 416.42
As a European Pharmacopoeia (EP) standard impurity of nimodipine, this compound has a well-defined structure and stable properties, and can be directly used for the quality testing of nimodipine active pharmaceutical ingredients and formulations. Its presence helps analyze the formation mechanism of by-products during reactions such as ester group introduction and nitro substitution in nimodipine synthesis, optimizing the production process to ensure drug quality meets international pharmacopoeia standards. Meanwhile, as a standard reference, it can improve the accuracy and reliability of impurity detection methods.
Quality Control: Used as an EP standard impurity reference for system suitability tests in HPLC, LC-MS, and other detection methods, verifying whether the content of this impurity in nimodipine meets the limit requirements specified by the EP;
Drug Development: In generic drug research, used to compare the impurity profiles of original drugs, ensuring the quality consistency between generic and original drugs;
Process Optimization: Guiding the optimization of key reaction conditions such as nitration and esterification in the synthesis route by analyzing the content of this impurity, reducing impurity generation.
Nimodipine is a dihydropyridine calcium channel blocker clinically used for treating cerebral vasospasm, ischemic brain injury, and other diseases. During its synthesis, if reaction conditions are not properly controlled, such as the reagent ratio in esterification reactions or the temperature in nitration reactions, impurities with abnormal nitrobenzene substitution and ester group structures (such as Impurity A) are likely to be generated. The European Pharmacopoeia has strict limits for nimodipine impurities, making research on this impurity an important part of international quality control for nimodipine.
Current research focuses on:
Detection Method Optimization: Using EP-recommended HPLC-UV or LC-MS/MS techniques to improve the detection sensitivity and specificity of this impurity, enabling trace detection;
Synthesis Process Improvement: Reducing impurity generation by adjusting parameters such as reaction temperature and catalyst type, and developing high-purity impurity synthesis routes to meet quality research needs;
Stability Studies: Investigating the degradation behavior of this impurity under conditions of light, high temperature, high humidity, etc., evaluating its impact on the stability of nimodipine formulations;
Toxicological Evaluation: Studying the potential toxicity of this impurity through in vitro cell experiments and animal models, providing data support for establishing safe limits.





 China
China