N-Nitroso Desmethyl Rizatriptan
Product Code:N031223
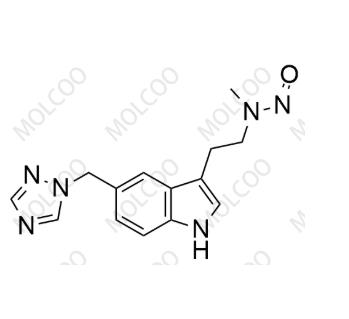
English Name:N-Nitroso Desmethyl Rizatriptan
English Alias:N-(2-(5-((1H-1,2,4-triazol-1-yl)methyl)-1H-indol-3-yl)ethyl)-N-methylnitrous amide
CAS No.:[Not Available]
Molecular Formula:C₁₄H₁₆N₆O
Molecular Weight:284.32
High-Purity Reference Standard:Confirmed by HPLC (≥99.0%), NMR (1H, 13C), HRMS, and elemental analysis, suitable for precise analysis of nitrosamine impurities in Rizatriptan.
Stability Assurance:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.2% in acetonitrile-water solution within 6 months.
Quality Control Testing:Used for UPLC-MS/MS detection of N-nitroso desmethyl impurities in Rizatriptan API and formulations, controlling content to meet ICH M7 standards (genotoxic impurity limits).
Process Optimization Research:Monitors N-Nitroso Desmethyl Rizatriptan formed by nitrosation side reactions during Rizatriptan synthesis or storage, reducing generation by >60% by adjusting reaction temperature (e.g., 5-10℃) and avoiding nitrite additives.
Method Validation:Serves as a standard for developing nitrosamine impurity detection methods, verifying UPLC resolution (≥3.0) and LOD (0.005 ng/mL).
Rizatriptan, a 5-HT receptor agonist for migraine treatment, may generate N-Nitroso Desmethyl Rizatriptan as a potential genotoxic impurity (GTI). This impurity can form during synthesis via reactions between desmethyl Rizatriptan and nitrites or through spontaneous nitrosation under acidic or high-temperature storage conditions. Its nitrosamide group is mutagenic. With stricter FDA and EMA regulations on GTIs, studying such impurities is crucial for ensuring drug safety.
Detection Technology:UPLC-MS/MS with C18 column (1.7μm) and 0.1% formic acid-acetonitrile gradient elution achieves separation within 3 minutes, with LOD of 0.002 ng/mL for trace nitrosamine analysis.
Formation Mechanism:Formed by reaction of desmethyl Rizatriptan with sodium nitrite under acidic conditions (pH<4); optimizing reaction pH (neutral) and adding antioxidants (e.g., ascorbic acid) inhibits its formation.
Safety Evaluation:In vitro Ames tests show mutagenicity; the permitted daily intake (TTC value) is determined as 1.5 μg/day, requiring strict content control. Accelerated stability testing is ongoing to monitor nitrosation rates under different humidity and light conditions.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!