N-Nitroso Desmethyl Zolmitriptan
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Number: Z004035
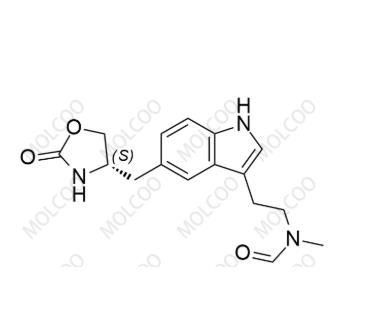
English Name: N-Nitroso Desmethyl Zolmitriptan
English Alias: (S)-N-methyl-N-(2-(5-((2-oxooxazolidin-4-yl)methyl)-1H-indol-3-yl)ethyl)formamide
CAS Number: None
Molecular Formula: C₁₆H₁₉N₃O₃
Molecular Weight: 301.34
As a nitroso impurity of desmethyl zolmitriptan, this compound has the following advantages:
Well-defined with distinct chiral features: Retains zolmitriptan’s indole core, oxazolidinone side chain, and (S)-chirality, with key difference of nitroso (-N=O) substitution on indole ethylamine. Nitroso-indole conjugation enables clear differentiation from parent drug via chiral HPLC/LC-MS as a specific impurity marker;
High stability and traceability: Rigid indole structure and nitroso chemical properties ensure stability under neutral conditions. As an oxidation product from desmethyl zolmitriptan reacting with nitrites, it directly reflects nitrosation risks during storage, improving quality tracing accuracy;
High detection sensitivity: UV absorption (250-270nm) from nitroso-indole conjugation, combined with characteristic mass response (m/z 302 [M+H]⁺), enables trace analysis (ppb level) via LC-MS/MS, compatible with indole-based antimigraine nitroso impurity systems.
Pharmaceutical quality control: Used as an impurity reference standard to quantify N-Nitroso Desmethyl Zolmitriptan in zolmitriptan APIs/formulations, ensuring nitrosation-derived impurities meet quality standards during production/storage;
Stability evaluation: Monitoring impurity levels under varying storage conditions to assess nitrosation risks and guide packaging/storage recommendations;
Toxicological risk assessment: Supporting studies on potential nitroso impurity toxicity to guide zolmitriptan impurity limit setting.
Zolmitriptan, a 5-HT agonist, has an active metabolite desmethyl zolmitriptan. During production or storage, desmethyl zolmitriptan may react with nitrosating agents to form N-nitroso derivatives like N-Nitroso Desmethyl Zolmitriptan. Due to potential toxicity of nitroso compounds, their control is critical for zolmitriptan quality assurance.
Current research focuses on:
Analytical method validation: Developing UPLC-MS/MS assays with chiral columns for baseline separation of impurity, parent drug, and metabolites, achieving 0.1 ppb detection limits;
Nitrosation kinetics: Studying impurity formation under varying nitrite/pH conditions to clarify amine nitrosation mechanisms of desmethyl zolmitriptan;
Formulation stabilization: Developing anti-nitrosation packaging or antioxidants to minimize impurity formation in zolmitriptan products;
Toxicity testing: Conducting in vitro genotoxicity assays (e.g., comet assay) to support scientifically based impurity limit recommendations
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com