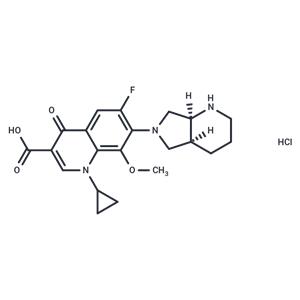
| Name | Moxifloxacin hydrochloride |
| Description | Moxifloxacin hydrochloride (BAY12-8039 HCl) is a fourth generation fluoroquinolone with expanded activity against gram-positive bacteria as well as atypical pathogens. Moxifloxacin has been linked to mild ALT elevations during therapy and to rare instances of idiosyncratic acute liver injury with symptoms and jaundice. |
| In vitro | In a mouse model simulating human disease, treatment with Moxifloxacin combined with RIF (Rifampicin)/PZA (Pyrazinamide) shortened the therapy duration by two months compared to the standard regimen of INH (Isoniazid)/RIF/PZA. Similarly, biweekly administration of Rifampicin/Moxifloxacin/PZA led to stable cure after four months, whereas daily treatment with Rifampicin/INH/PZA resulted in cure after six months. |
| In vivo | Moxifloxacin exhibits a broad spectrum of antibacterial activity both in vitro and clinically against various pathogens, including Staphylococcus aureus, Streptococcus pneumoniae, Streptococcus pyogenes, Haemophilus influenzae, H. parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis, Chlamydophila pneumoniae, and Mycoplasma pneumoniae. It functions by stabilizing the DNA-drug-enzyme complex, thereby inhibiting ATP-dependent topoisomerase II (DNA gyrase) and topoisomerase IV, critical enzymes for bacterial DNA replication, transcription, repair, and recombination. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | H2O : 15 mg/mL (34.26 mM), Sonication is recommended.
DMSO : 88 mg/mL (200.96 mM), Sonication is recommended.
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 3.3 mg/mL (7.54 mM), Sonication is recommended.
|
| Keywords | Topo IV | Topo II | sinusitis | pneumonia | Moxifloxacin Hydrochloride | Moxifloxacin hydrochloride | Moxifloxacin | Inhibitor | inhibit | exacerbations | community-acquired | chronic | bronchitis | BAY128039 | BAY 12-8039 | BAY 128039 | Bacterial | antimicrobial | Antibiotic | acute | 8-methoxyquinolone |
| Inhibitors Related | Neomycin sulfate | Dehydroacetic acid sodium | Ampicillin sodium | Methyl anthranilate | Doxycycline (hyclate) | Kanamycin sulfate | Urethane | Sulfamethoxazole sodium | Doxycycline | EDTA copper(II) disodium salt | Isoeugenol | Dimethyl sulfoxide |
| Related Compound Libraries | DNA Damage & Repair Compound Library | Bioactive Compound Library | Approved Drug Library | Drug-induced Liver Injury (DILI) Compound Library | ReFRAME Related Library | Drug Repurposing Compound Library | Cardiotoxicity Compound Library | NO PAINS Compound Library | FDA-Approved Drug Library | Anti-Aging Compound Library | Immunology/Inflammation Compound Library | Bioactive Compounds Library Max |

 United States
United States