| Name | Lopinavir |
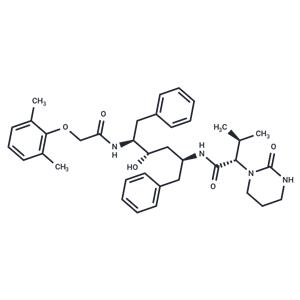
| Description | Lopinavir (ABT-378) is a peptidomimetic HIV protease inhibitor effective against HIV protease with the Val 82 mutation. It is less affected by serum protein binding compared to the structurally related drug ritonavir. |
| In vitro | Administering Lopinavir (10 mg/kg, p.o.) to rats resulted in a maximum concentration (Cmax) of 0.8 μg/mL, with the drug's bioavailability being 25%. |
| In vivo | Lopinavir is an effective inhibitor of Rh123, with an IC50 value of 1.7 mM for Caco-2 cell monolayers. It binds to mutant HIV proteases (V82A, V82T, and V82F) with Ki values of 4.9, 3.7, and 3.6 pM, respectively. At a concentration of 0.5 nM, Lopinavir inhibits the activity of wild-type HIV protease by 93%. It also inhibits HIV protease activity in MT4 cells both in the presence and absence of 50% HS, with EC50 values of 102 nM and 17 nM, respectively. In liver microsomes, Lopinavir is converted to primary metabolites M-3 and M-4, a process that is NADPH-dependent. After treating LS 180V cells with Lopinavir for 72 hours, there is a reduction in intracellular Rh123 content and induction of P-glycoprotein immunoreactive protein and mRNA levels. Lopinavir exhibits an IC50 of 9.4 nM against subtype C clone C6. When acting on human liver microsomes, Lopinavir shows an IC50 of 7.3 mM against CYP3A and exerts weak inhibition on human CYP1A2, 2B6, 2C9, 2C19, and 2D6. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | Ethanol : 116 mg/mL (184.5 mM)
DMSO : 116 mg/mL (184.5 mM)
H2O : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | inhibit | coadministration | SARS coronavirus | Lopinavir | mutant | Inhibitor | wild-type | HIV | Human immunodeficiency virus | SARS-CoV | infection | ABT378 | replication | HIV Protease | ABT 378 |
| Inhibitors Related | Stavudine | 5-Fluorouracil | Emtricitabine | Kaempferol | Dolutegravir intermediate-1 | Dextran sulfate sodium salt (MW 4500-5500) | Hydroxychloroquine | Lamivudine | Decanedioic acid | Tenofovir |
| Related Compound Libraries | Bioactive Compound Library | Approved Drug Library | EMA Approved Drug Library | Anti-Viral Compound Library | Drug Repurposing Compound Library | Inhibitor Library | FDA-Approved Drug Library | Clinical Compound Library | Bioactive Compounds Library Max | Human Metabolite Library |

 United States
United States