| CAS: | 87-91-2 |
| MF: | C8H14O6 |
| MW: | 206.19 |
| EINECS: | 201-783-3 |
| Product Categories: | Chiral Compound;Asymmetric Synthesis;Chiral Building Blocks;Esters (Chiral);Synthetic Organic Chemistry;CHIRAL CHEMICALS;Pharmaceutical Intermediates;Chiral Compounds;chiral;Hydroxy Acids & Deriv.;bc0001 |
| Mol File: | 87-91-2.mol |
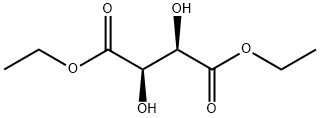 |
|
| L(+)-Diethyl L-tartrate Chemical Properties |
| Melting point | 17 °C |
| alpha | 7.5 º (neat) |
| Boiling point | 280 °C (lit.) |
| density | 1.204 g/mL at 25 °C (lit.) |
| vapor pressure | 0.402Pa at 25℃ |
| refractive index | n20/D 1.446(lit.) |
| FEMA | 2378 | DIETHYL TARTRATE |
| Fp | 200 °F |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | Chloroform (Slightly), Water (Slightly) |
| pKa | 11.61±0.20(Predicted) |
| form | Viscous Liquid |
| color | Clear |
| Odor | at 100.00 %. mild fruity wine caramellic |
| Odor Type | fruity |
| optical activity | [α]20/D +8.5°, neat |
| Water Solubility | insoluble |
| Merck | 14,3855 |
| JECFA Number | 622 |
| BRN | 1727145 |
| InChIKey | YSAVZVORKRDODB-PHDIDXHHSA-N |
| LogP | 0.2 at 25℃ |
| CAS DataBase Reference | 87-91-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Diethyl tartrate(87-91-2) |
| EPA Substance Registry System | Butanedioic acid, 2,3-dihydroxy- (2R,3R)-, 1,4-diethyl ester (87-91-2) |
| L(+)-Diethyl L-tartrate Usage And Synthesis |
| Chemical Properties | Diethyl tartrate has a mild, fruity, wine aroma. |
| Chemical Properties | Colorless to light yellow liqui |
| Occurrence | The d-isomer has not been reported found in nature; the l-isomer and the racemic form are of little importance. Reported found in sherry, white and red wine. |
| Uses | (+)-Diethyl L-tartrate is used as a chiral auxiliary in the Sharpless enantioselective epoxidation of allylic alcohols, for Sharpless-type enantioselective oxidation of sulfides to sulfoxides and as a chiral auxiliary in the enantioselective construction of cyclopropanes from allylic alcohols by an asymmetric Simmons-Smith reaction. It is also used as a chiral reagent in a host of chemical reactions, such as the synthesis of isoquinoline alkaloids and arundic acid, which has been used in acute ischemic stroke therapy. |
| Uses | (+)-Diethyl L-tartrate can be used in the synthesis of biologically active compounds such as (+)-altholactone, (-)-aspicilin, (+)-monomorine I and (+)-(1R,2R,3S,6S)-3,6-di-O-methyl conduritol-E. |
| Uses | Diethyl L-(+)-Tartrate is used as a chiral reagent in a host of chemical reactions, such as the synthesis of isoquinoline alkaloids and arundic acid, which has been used in acute ischemic stroke thera py. |
| General Description | Made from natural tartaric acid |
| Flammability and Explosibility | Non flammable |
Packing &shipping&Payment
Packing:25kg/drum
Shipping:by sea or by air
Payment:T/T,western union,moneygram
Packaging Details drum
Port:Tianjin
Lead Time :
| Quantity(Kilograms) | 1 - 10000 | >10000 |
| Est. Time(days) | 5 | To be negotiated |

 Company information
Company information
Hebei Mojin Biotechnology Co., Ltd, Our company is a professional in 4'-Methylacetophenone,Levamisole hydrochloride ,N-Methylformamide and other chemical reagents research and development production enterprises. Our business covers more than 30 countries, most of the big customers come from Europe, America and other countries in the world, we can guarantee the quality and price. In recent decades, with the efforts of all employees, we have established many cooperative companies in shandong, henan, guangdong and other places. Our corporate purpose is based on the market, enhance the strength, take the road of scientific and environmental sustainable development, relying on the country. Technology r & d center, increase the investment in r & d, based on the domestic market, expand the international market, manufacturing quality products, sincere service to the society, into a modern, ecological, scientific and technological enterprise world.
 Advantage
Advantage
In stock
