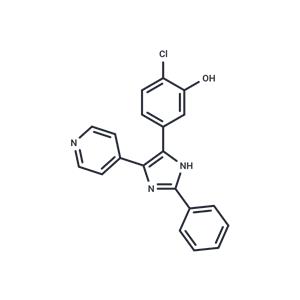
| Kinase Assay | Akt kinases assay: Akt kinases are assayed by a GSK-derived biotinylated peptide substrate. The extent of peptide phosphorylation is determined by Homogeneous Time Resolved Fluorescence (HTRF) using a lanthanide chelate (Lance)-coupled monoclonal antibody specific for the phosphopeptide in combination with a streptavidin-linked allophycocyanin (SA-APC) fluorophore which will bind to the biotin moiety on the peptide. When the Lance and APC are in proximity, a non-radiative energy transfer takes place from the Lance to the APC, followed by emission of light from APC at 655 nm. Working Solution: 100X protease inhibitor cocktail (PIC): 1 mg/mL benzamidine, 0.5 mg/mL pepstatin, 0.5 mg/mL leupeptin, 0.5 mg/mL aprotinin; 10X assay buffer: 500 mM HEPES, pH7.5, 1% PEG, 16.6 mM EDTA, 1 mM EGTA, 1% BSA, 20 mM 9-glycerol phosphate; Quench buffer 50 mM HEPES pH 7.3, 16.6 mM EDTA, 0.1% BSA, 0.1% Triton X-100, 0.17 nM labeled monoclonal antibody, 0.0067 mg/mL SA-APC; ATP/MgCl2 working solution: 1X Assay buffer, 1 mM DTT, 1X PIC, 5% glycerol, active Akt; Peptide working solution: 1X Assay buffer, 1 mM DTT, 1X PIC, 5% glycerol, 2 TM GSK biotinylated peptide. The reaction is assembled by adding 16 μL of ATP/MgCl2 working solution to the appropriate wells. MK-2206 or vehicle (1.0 μL) is added followed by 10 μL of peptide working solution. The reaction is started by adding 13 μL of the enzyme working solution and mixing. The reaction is allowed to proceed for 50 min and then stopped by the addition of 60 μL HTRF quench buffer. The stopped reactions are incubated at room temperature for at least 30 min and then read in the instrument. |

 United States
United States