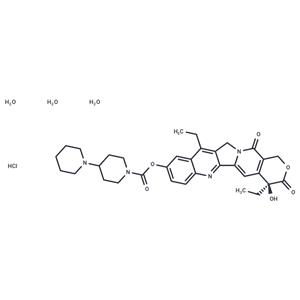
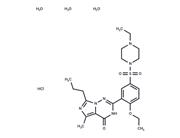
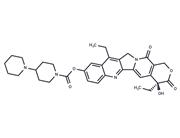
| Name | Irinotecan hydrochloride trihydrate |
| Description | Irinotecan hydrochloride trihydrate (CPT-11 HCl Trihydrate) keeps DNA from unwinding by inhibiting topoisomerase 1. |
| Cell Research | Exponentially growing cells (LoVo and HT-29 cells) are seeded in 20 cm2 Petri dishes with an optimal cell number for each cell line (2 × 104 for LoVo cells, 105 for HT-29 cells). They are treated 2 days later with increasing concentrations of Irinotecan or SN-38 for one cell doubling time (24 hours for LoVo cells, 40 hours for HT-29 cells). After washing with 0.15 M NaCl, the cells are further grown for two doubling times in normal medium, detached from the support with trypsin-EDTA and counted in a hemocytometer. The IC50 values are then estimated as the Irinotecan or SN-38 concentrations responsible for 50% growth inhibition as compared with cells incubated without Irinotecan or SN-38. (Only for Reference) |
| In vitro | In the liver, stomach, duodenum, and colon, a single dose of Irinotecan significantly increases the covalent binding of topoisomerase I to DNA. Compared to the control group, there is a marked increase in DNA strand breaks within the colonic mucosal cells in the Irinotecan-treated group. In COLO320 xenografts, Irinotecan induces a 92% maximum growth inhibition. |
| In vivo | In LoVo and HT-29 cell lines, Irinotecan induces a similar number of cleavable complexes at IC50 concentrations, with no significant difference observed between the two. The formation of cleavable complexes by SN-38 is concentration-dependent and consistent across both cell lines. Notably, intracellular accumulation of Irinotecan differs significantly, with consistently higher levels in HT-29 cells compared to LoVo cells. Carboxylesterase activation of Irinotecan to SN-38 (primarily in the liver) facilitates interaction with its target, topoisomerase I, in plasma, intestines, and tumor tissues. The hydrolysis of Irinotecan’s lactone E-ring and SN-38 is reversible in aqueous solution, with the interconversion of their carboxylate and lactone forms dependent on temperature and pH. For the same concentration of SN-38 glucuronide and Irinotecan in tumor and normal tissues, the yield of SN-38 via β-glucuronidase mediation exceeds that of SN-38 produced from Irinotecan. In SCLC cell lines, Irinotecan exhibits markedly greater activity than in NSCLC cell lines, while tissue histology does not reveal significant differences in SN-38 efficacy. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice/Shipping at ambient temperature. |
| Solubility Information | H2O : 1 mg/mL (1.48 mM), Sonication is recommended.
10% DMSO+40% PEG300+5% Tween 80+45% Saline : 2 mg/mL (2.95 mM), Sonication is recommended.
Ethanol : 7 mg/mL (10.34 mM), Sonication is recommended.
DMSO : 45 mg/mL (66.45 mM), Sonication is recommended.
|
| Keywords | Topoisomerase | Topo I | Irinotecan hydrochloride trihydrate | Irinotecan hydrochloride Trihydrate | Irinotecan hydrochloride | Irinotecan Hydrochloride | Irinotecan HCl | Inhibitor | inhibit | CPT-11 hydrochloride | CPT-11 HCl | Autophagy | (+)-Irinotecan hydrochloride |
| Inhibitors Related | Stavudine | Aceglutamide | Cysteamine hydrochloride | Sodium 4-phenylbutyrate | Hydroxychloroquine | Guanidine hydrochloride | 1,4-Naphthoquinone | Valproic Acid | Paeonol | Naringin | Alginic acid | Gefitinib |
| Related Compound Libraries | Bioactive Compound Library | EMA Approved Drug Library | Autophagy Compound Library | Anti-Cancer Clinical Compound Library | Drug Repurposing Compound Library | Inhibitor Library | Anti-Cancer Approved Drug Library | FDA-Approved Drug Library | Anti-Aging Compound Library | Bioactive Compounds Library Max | Anti-Cancer Active Compound Library | Anti-Cancer Drug Library |

 United States
United States