Product No.:I003056
English Name:Imatinib Impurity 56
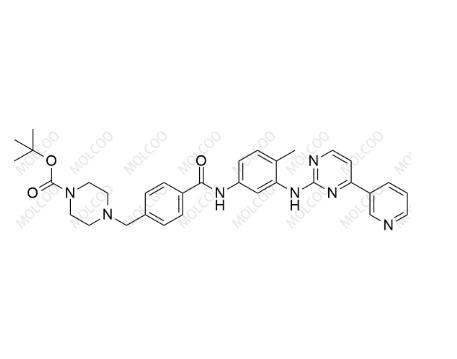
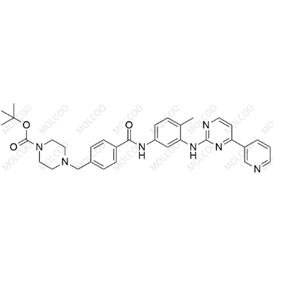
English Alias:tert-butyl 4-(4-((4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)phenyl)carbamoyl)benzyl)piperazine-1-carboxylate
CAS No.:1076199-23-9
Molecular Formula:C₃₃H₃₇N₇O₃
Molecular Weight:579.69
High-purity standard substance, suitable for impurity analysis, quality control, and methodology validation of Imatinib-related drugs. With a clear structure and good stability, it meets the strict requirements for qualitative and quantitative analysis of impurities in drug research, development, and production.
Mainly used for impurity research of Imatinib active pharmaceutical ingredients (APIs) and formulations, such as impurity limit detection, chromatographic method development and validation, and drug stability investigation. In the pharmaceutical industry, it can be used as a reference standard to evaluate the rationality of drug production processes, ensuring drug safety and quality controllability.
Imatinib is a tyrosine kinase inhibitor widely used in the treatment of chronic myeloid leukemia (CML) and other diseases. The control of drug impurities is an important part of the drug quality system, as the presence of impurities may affect the efficacy and safety of drugs. As a related impurity of Imatinib, Imatinib Impurity 56 has a structure similar to the active ingredient. Therefore, the study of this impurity helps optimize the drug production process, reduce impurity content, and ensure drug quality.
At present, research on Imatinib impurities mainly focuses on the synthesis, separation, characterization, and toxicity evaluation of impurities. Studies on Imatinib Impurity 56 are mostly combined with drug analysis techniques (such as HPLC, LC-MS, etc.) to establish impurity detection methods and formulate quality standards. With the improvement of drug regulatory requirements, the research on this impurity has gradually deepened, including its formation mechanism during drug storage and potential impact on drug efficacy. Relevant studies provide a scientific basis for the quality improvement and clinical application safety of Imatinib.
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17320513646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!





 China
China