1. Product information
| Product Name: | DM1-SMCC |
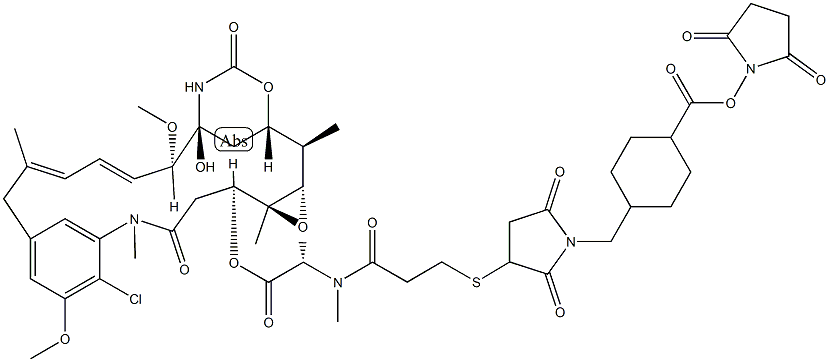
| Synonyms: | DM1-SMCC;N2'-Deacetyl-N2'-[3-[[1-[[4-[[(2,5-dioxo-1-pyrrolidinyl)oxy]carbonyl]cyclohexyl]methyl]-2,5-dioxo-3-pyrrolidinyl]thio]-1-oxopropyl]-maytansine;SMCC-DM1;DM1-SMC;SMCC-DM1 (DM1-SMCC);SMCC-MDC;Maytansine, N2'-deacetyl-N2'-[3-[[1-[[4-[[(2,5-dioxo-1-pyrrolidinyl)oxy]carbonyl]cyclohexyl]methyl]-2,5-dioxo-3-pyrrolidinyl]thio]-1-oxopropyl]-;DM1 SMCC; DM1SMCC |
| CAS: | 1228105-51-8 |
| MF: | C51H66ClN5O16S |
| MW: | 1072.61 |
| EINECS: |
|
| Product Categories: | Pharmaceutical |
| Mol File: | 1228105-51-8.mol |
 |
|
| DM1-SMCC Chemical Properties |
| Melting point | >166°C (dec.) |
| density | 1.41±0.1 g/cm3(Predicted) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| pka | 9.82±0.70(Predicted) |
| form | Solid |
| color | White to Off-White |
|
| DM1-SMCC Usage And Synthesis |
| In vivo | The antitumor effect of murine/human chimeric CD138-specific monoclonal antibody nBT062 conjugated with highly cytotoxic maytansinoid derivatives against multiple myeloma (MM) cells were investigated in vitro and in vivo. The in vivo activity of BT062SPDB-DM4, BT062-SMCC-DM1, and BT062-SPP-DM was examined in murine MM cell xenograft model of human and severe combined immunodeficient (SCID) mice bearing implant bone chips injected with human MM cells (SCID-hu model). The in vivo efficacy of nBT062-SPDB-DM4, nBT062-SMCC-DM1, and nBT062-SPP-DM1 was next evaluated in SCID mice bearing established CD138-positive MOLP-8 human MM cells. A single i.v. administration of the immunoconjugates caused significant dose-dependent tumor growth inhibition and tumor regression at concentrations that were well tolerated, evidenced by stable body weight. nBT062SPDB-DM4 was the most active conjugate tested in this model (Fig. 6A ). In addition, weekly dosing of the nBT062-SMCC-DM1 (six doses of 13.8 μg/kg) completely blocked tumor growth during the dosing period (Supplementary Fig. S6A). In summary, nBT062-SMCC-DM1, nBT062-SPDB-DM4, and nBT062-SPP-DM1 have in vitro and in vivo antitumor activity against CD138positive MM cells and can overcome the protective effects of cytokines and BMSCs Clin Cancer Res. 2009 Jun 15;15(12):4028-37. https://pubmed.ncbi.nlm.nih.gov/19509164/ |
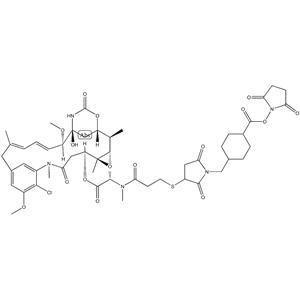
| Description | DM1-SMCC is a drug-linker conjugate composed of a potent microtubule-disrupting agent DM1 and a linker SMCC. |
| Uses | SMCC-DM1 is a mertansine drug (DM1) with a reactive linker SMCC to make antibody drug conjugate. DM1 (mertansine), a thiol-containing maytansinoid, is a potent microtubule-disrupting agent. |
| in vitro | In particular, the metabolic fate in cells of huC242-maytansinoid conjugates containing either a disulfide linker (huC242-SPDB-DM4) or a thioether linker (huC242-SMCC-DM1) was examined. The disulfide-linked antibody-maytansinoid conjugate, huC242-SPDBDM4, and the thioether-linked conjugate, huC242-SMCC-DM1, were first assayed for their cytotoxic potency against antigen-positive COLO 205 cells and antigen-negative Namalwa cells (both sensitive to maytansine with IC50 values of ~30 to 60 pmol/L for both cell lines) using an 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)–based assay. The conjugates displayed similar potencies with IC50 values of 40 pmol/L against COLO 205 cells and 20 to 80 nmol/L against Namalwa cells upon a 4-day exposure of the cells to the conjugates ( Fig. 1A ). To examine the fate of the maytansinoid drug upon incubation of target cells with an antibody-maytansinoid conjugate, conjugates with maytansinoids were prepared that were 3H-labeled at the C-20 methoxy group (see Fig. 5). Radiolabeled maytansinoid conjugates, huC242-SPDB-[3H]DM4 (250 mCi/mmol) and huC242-SMCC-[3H]DM1 (214 mCi/mmol), exhibit in vitro cytotoxicities similar to nonradiolabeled conjugate samples (data not shown). The two major metabolites, S-methyl-DM4 and lysine-Nε-SMCC-DM1, were synthesized and tested for their in vitro cytotoxicity against COLO 205 cells and Namalwa cells. Lysine-Nε-SMCC-DM1 was ~105-fold less potent against both cell lines with an IC50 value of 0.1 μmol/L (data not shown). Accumulation of the lysine-Nε-SMCC-DM1 metabolite from the noncleavable conjugate is coincident with the observed formation of the potent S-methyl-DM4, DM4, and lysine-Nε-SPDB-DM4 from the cleavable conjugate. This suggests that the lysineNε-SMCC-DM1 metabolite is as potent as the metabolites from the cleavable conjugate when delivered intracellularly and that all of the maytansinoid metabolites are active when produced in the cell. Cancer Res. 2006 Apr 15;66(8):4426-33. https://pubmed.ncbi.nlm.nih.gov/16618769/ |
2. Packaging
For powders: normal is 25kgs/Drum or bag, or larger/smaller package as request.
For liquids: normal 25kgs/drum, 180-300kgs/bucket, or IBC, determined by the nature of the product.
Or smaller package 1kg/bottle, 10kgs/bottle as request.


3. Shipping

4. Contact information
For more details, pls contact us freely.
Email address : elin@fdachem.com
Mob: 86 13613820652
WhatsApp/Skype/Wechat/LINE: 86 13613820652


 China
China