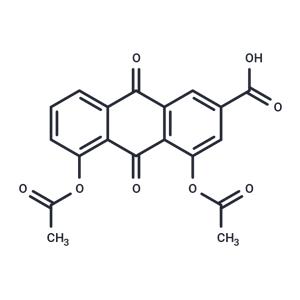
| Name | Diacerein |
| Description | Diacerein (Fisiodar) is a prodrug which is metabolized to rhein. It is currently approved in France for the treatment of osteoarthritis although the use of diacerein is restricted due to the side effects including sevre diarrhea [L780]. Diacerein is under investigation for the treatment of Insulin Resistance, Diabetes Mellitus (Type 2), and Diabetes-Related Complications. |
| Kinase Assay | In Vitro Growth Inhibition Assay: Stock solution of Cytarabine is prepared in absolute ethanol, and serial dilutions of Cytarabine are prepared. CCRF-CEM cells are suspended in RPMI medium supplemented with 10% FBS, 0.1% gentamicin, and 1% sodium pyruvate. The cells are suspended in their respective media to give 10 mL volumes of cell suspension at a final density of 3-6 × 104 cells/mL. Appropriate volumes of Cytarabine solution are transferred to the cell suspensions, and incubation is continued for 72 hours. The cells are spun down and resuspended in fresh Cytarabine -free medium, and final cell counts are determined. The data are analyzed by sigmoidal curve fitting of the cell count versus Cytarabine concentration, and the results are expressed as the IC50 (Cytarabine concentration that inhibits cell growth to 50% of the control value). |
| In vitro | In ovariectomized rats, daily administration of 100 mg/kg of Diacerein significantly prevented bone loss and reduced levels of serum alkaline phosphatase, as well as diminished the excretion of hydroxyproline in urine. The same daily dose of Diacerein notably suppressed paw edema and the increase of serum mucoproteins in adjuvant-induced arthritic rats. In these rats, the combined use of 30 mg/kg Diacerein with 3 mg/kg Naproxen daily exhibited more pronounced anti-inflammatory activity than Naproxen alone. Moreover, at the lesion site on the lateral tibial plateau in Merino sheep, 25 mg/kg Diacerein reduced the thickness of the cartilage and subchondral bone. |
| In vivo | Diacerein significantly reduces the activity of MMP-13 and cathepsin K in osteoclasts. It effectively blocks the effects of IL-1β on both the differentiation process of osteoclasts and the survival of mature osteoclasts. Diacerein notably inhibits IL-1β production induced by lipopolysaccharides in synovial tissues and cartilage. Dose-dependently, diacerein decreases the generation of MMP-13 induced by interleukin-1-beta (IL-1β) in osteoarthritic cartilage. At 1 μM, diacerein exhibits a notably lower inhibitory effect on cartilage synthesis compared to a medium containing only LPS. At this concentration, diacerein also reduces nitric oxide (NO) release in the synovial and cartilage culture mediums and increases IL-1ra levels in the cartilage culture medium. At 10 μM, diacerein enhances the expression of TGF-β1 and TGF-β2 in cultured bovine articular chondrocytes. |
| Storage | Powder: -20°C for 3 years | In solvent: -80°C for 1 year | Shipping with blue ice. |
| Solubility Information | DMSO : 6.25 mg/mL (16.97 mM), Sonication is recommended.
Ethanol : < 1 mg/mL (insoluble or slightly soluble)
H2O : < 1 mg/mL (insoluble or slightly soluble)
|
| Keywords | Interleukin Related | Inhibitor | Diacerein | inhibit |
| Related Compound Libraries | Bioactive Compound Library | Traditional Chinese Medicine Monomer Library | Selected Plant-Sourced Compound Library | Natural Product Library | Drug Repurposing Compound Library | Natural Product Library for HTS | Anti-Aging Compound Library | Bioactive Compounds Library Max |

 United States
United States