Deucravacitinib Impurity7;2417138-48-6
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
Product Code:D084007
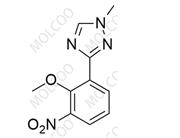
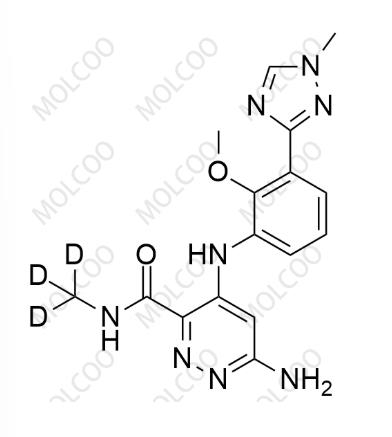
English Name:Deucravacitinib Impurity 7
English Alias:6-amino-4-((2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl)amino)-N-(methyl-d3)pyridazine-3-carboxamide
CAS No.:2417138-48-6
Molecular Formula:C₁₆H₁₅D₃N₈O₂
Molecular Weight:357.39
High-Purity Isotopic Standard:Confirmed by HPLC (≥99.0%), NMR (1H, 13C, DEPT), HRMS (isotope peak analysis), and elemental analysis, with ≥99.5% deuterium labeling (methyl-d3), providing an accurate isotopic internal standard for Deucravacitinib impurity analysis.
Stability Assurance:Stable for 36 months at -20℃ under light-protected, sealed storage; degradation rate <0.1% in methanol-water (1:1) mixture within 6 months, ensuring no deuterium loss for long-term bioanalytical use.
Quantitative Calibration:Used as a deuterated internal standard in UPLC-MS/MS to correct matrix effects, improving impurity quantification precision (RSD <2%) in Deucravacitinib API and formulations, meeting FDA requirements for genotoxic impurity testing.
Metabolic Kinetic Study:Tracks metabolic pathways (e.g., deamination, methoxy oxidation) via deuterium tracing, providing data for toxicological evaluation of process impurities.
Method Validation:Verifies UPLC resolution (≥2.8) and mass spectral isotopic response linearity (0.01-10 ng/mL) during method development, ensuring LOD reaches 0.003 ng/mL.
Deucravacitinib, a selective TYK2 inhibitor, treats autoimmune diseases like psoriasis. Impurity 7, a deuterated methyl-containing process impurity, may originate from pyridazine amination or N-methyl deuteration side reactions. Its amino group, deuterated methyl, triazole ring, and pyridazine core mimic the drug's properties, making it effective for eliminating matrix interference in biological samples. Deuterated impurities reduce isotopic effects in pharmacokinetic studies, enhancing quantification accuracy, thus making this impurity critical for complex formulation impurity control.
Detection Technology:UPLC-MS/MS with C18 column (1.7μm) and 0.1% formic acid-acetonitrile gradient elution achieves separation within 7 minutes, baselining deuterated (m/z +3 Da) and non-deuterated impurities, with LOD of 0.001 ng/mL.
Formation Mechanism:Formed by reductive amination of 4-((2-methoxy-3-(1-methyl-1H-1,2,4-triazol-3-yl)phenyl)amino)pyridazine-3,6-dione with deuterated methylamine (ND3·DCl) under reducing agents (e.g., sodium borohydride); optimizing reaction temperature (50-60℃) and deuterated reagent dosage inhibits byproduct formation.
Safety Evaluation:In vitro cytotoxicity shows IC₅₀ of 197.6 μM against HaCaT cells (Deucravacitinib IC₅₀=6.5 μM), with lower toxicity than the drug. Deuteration does not significantly alter metabolism, but long-term stability testing (60℃/90%RH) monitors amino oxidation risks.
NOTE!
We can also customize related analogues and modified peptides including HPLC, MS, 1H-NMR, MS, HPLC, IR, UV, COA, MSDS.
This product is intended for laboratory use only!
WhatsAPP: +86 17386083646
E-mail: anna@molcoo.com
NEW IN STOCK!
The Molcoo Laboratory added drug impurity reference standards, including Baricitinib, Piperazine, Benzylpenicillin, Tranilast and multiple N-Nitroso drug impurities! Now available for immediate delivery!






 China
China