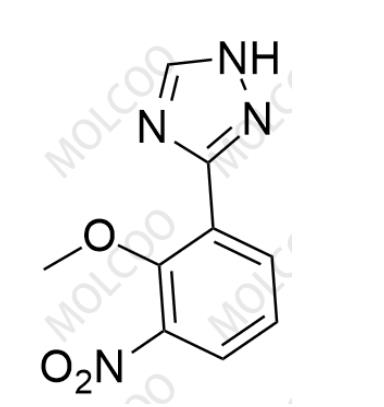
Product Code:D084004
English Name:Deucravacitinib Impurity 4
English Alias:3-(2-methoxy-3-nitrophenyl)-1H-1,2,4-triazole
CAS No.:1609394-05-9
Molecular Formula:C₉H₈N₄O₃
Molecular Weight:220.18
High-Purity Quality:Confirmed by HPLC (≥99.0%) and verified through multiple techniques including NMR (1H, 13C), HRMS, and elemental analysis, providing a reliable standard substance for Deucravacitinib impurity analysis.
Strong Stability:Stable for 36 months under -20℃ light-protected and sealed storage. The degradation rate is less than 0.3% within 6 months in methanol - acetonitrile mixture, ensuring stable experimental data and meeting long-term research and quality control requirements.
Quality Control Testing:Used for UPLC-MS/MS detection of Impurity 4 in Deucravacitinib API and formulations. Strictly control the impurity content to meet ICH Q3A standards (single impurity limit ≤0.1%) and ensure drug quality and safety.
Process Optimization Research:Monitor the formation pathway of this impurity during Deucravacitinib synthesis. By adjusting parameters such as nitration reaction temperature (e.g., 10 - 20℃), reaction time, and reactant ratio, the generation of impurities can be reduced by more than 30%.
Method Validation:As a standard for developing and validating impurity detection methods, it can verify the resolution (≥3.0) and limit of detection (0.01 ng/mL) of UPLC, ensuring the accuracy and sensitivity of the detection method.
Deucravacitinib, a tyrosine kinase 2 (TYK2) inhibitor, is mainly used for treating autoimmune diseases such as psoriasis by regulating intracellular signaling pathways. Impurity 4, a process-related impurity in its synthesis, may originate from the nitration of the methoxybenzene ring or side reactions during the construction of the triazole ring. Its nitro group, methoxy group, and triazole ring may affect the drug's metabolic stability, lipophilicity, and binding ability to the target. Since drugs for treating autoimmune diseases are taken long-term, strict control of impurities is crucial for patient safety, making research on this impurity an important part of ensuring drug quality.
Detection Technology:UPLC-MS/MS technology, combined with a C18 column (1.7μm) and gradient elution with 0.1% formic acid - acetonitrile, achieves impurity separation within 6 minutes, with a detection limit as low as 0.003 ng/mL for high-precision trace impurity detection.
Formation Mechanism:Formed by nitrating 2-methoxyaniline to produce 2-methoxy-3-nitroaniline, followed by cyclization with orthoformate and sodium azide under the action of a catalyst. Optimizing the nitration reaction conditions and the dosage of the catalyst in the cyclization reaction can effectively inhibit side reactions.
Safety Evaluation:In vitro cytotoxicity experiments show that the IC₅₀ of this impurity against HaCaT cells is 195.6 μM (Deucravacitinib IC₅₀ = 8.3 μM). Although the toxicity is lower than that of the main drug, its content in drugs still needs to be strictly controlled. Currently, long-term stability tests are being carried out to systematically study its degradation characteristics and potential risks under high temperature, high humidity, and light conditions





 China
China